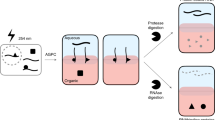
Abstract
Existing high-throughput methods to identify RNA-binding proteins (RBPs) are based on capture of polyadenylated RNAs and cannot recover proteins that interact with nonadenylated RNAs, including long noncoding RNA, pre-mRNAs and bacterial RNAs. We present orthogonal organic phase separation (OOPS), which does not require molecular tagging or capture of polyadenylated RNA, and apply it to recover cross-linked protein–RNA and free protein, or protein-bound RNA and free RNA, in an unbiased way. We validated OOPS in HEK293, U2OS and MCF10A human cell lines, and show that 96% of proteins recovered were bound to RNA. We show that all long RNAs can be cross-linked to proteins, and recovered 1,838 RBPs, including 926 putative novel RBPs. OOPS is approximately 100-fold more efficient than existing methods and can enable analyses of dynamic RNA–protein interactions. We also characterize dynamic changes in RNA–protein interactions in mammalian cells following nocodazole arrest, and present a bacterial RNA-interactome for Escherichia coli. OOPS is compatible with downstream proteomics and RNA sequencing, and can be applied in any organism.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE91 partner repository with the dataset identifier PXD009668. All sequencing data can be accessed through the European Nucleotide Archive, accession code PRJEB26736.
Change history
14 January 2019
In the supplementary information originally posted online, the Supplementary Note was missing.
23 May 2019
An amendment to this paper has been published and can be accessed via a link at the top of the paper
References
García-Mauriño, S. M. et al. RNA binding protein regulation and cross-talk in the control of AU-rich mRNA fate. Front. Mol. Biosci. 4, 71 (2017).
Müller-McNicoll, M. & Neugebauer, K. M. How cells get the message: dynamic assembly and function of mRNA-protein complexes. Nat. Rev. Genet. 14, (275–287 (2013).
Huntzinger, E. & Izaurralde, E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat. Rev. Genet. 12, 99–110 (2011).
Engreitz, J. M. et al. Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature 539, 452–455 (2016).
Wang, K. C. et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 472, 120–124 (2011).
Di Ruscio, A. et al. DNMT1-interacting RNAs block gene-specific DNA methylation. Nature 503, 371–376 (2013).
McHugh, C. A. et al. The Xist lncRNA interacts directly with SHARP to silence transcription through HDAC3. Nature 521, 232–236 (2015).
Hafner, M. et al. PAR-CliP—a method to identify transcriptome-wide the binding sites of RNA binding proteins. J. Vis. Exp. 2–6, 2034, https://doi.org/10.3791/2034 (2010).
Huppertz, I. et al. iCLIP: protein-RNA interactions at nucleotide resolution. Methods 65, 274–287 (2014).
Castello, A. et al. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell 149, 1393–1406 (2012).
Baltz, A. G. et al. The mRNA-bound proteome and its global occupancy profile on protein-coding transcripts. Mol. Cell 46, 674–690 (2012).
Beckmann, B. M. et al. The RNA-binding proteomes from yeast to man harbour conserved enigmRBPs. Nat. Commun. 6, 10127 (2015).
Sysoev, V. O. et al. Global changes of the RNA-bound proteome during the maternal-to-zygotic transition in Drosophila. Nat. Commun. 7, 12128 (2016).
Huang, R., Han, M., Meng, L. & Chen, X. Transcriptome-wide discovery of coding and noncoding RNA-binding proteins. Proc. Natl Acad. Sci. USA 115, E3879–E3887 (2018).
Bao, X. et al. Capturing the interactome of newly transcribed RNA. Nat. Methods 15, 213–220 (2018).
Jao, C. & Salic, A. Exploring RNA transcription and turnover in vivo by using click chemistry. Proc. Natl Acad. Sci. USA 105, 15779–15784 (2008).
Chomczynski, P. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162, 156–159 (1987).
Chomczynski, P. & Sacchi, N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nat. Protoc. 1, 581–585 (2006).
Wagenmakers, A. J., Reinders, R. J. & van Venrooij, W. J. Cross-linking of mRNA to proteins by irradiation of intact cells with ultraviolet light. Eur. J. Biochem. 112, 323–330 (1980).
Harvey, R. F. et al. Trans-acting translational regulatory RNA binding proteins. Wiley Interdiscip. Rev. RNA 9, e1465 (2018).
Van Nostrand, E. L. et al. Robust transcriptome-wide discovery of RNA-binding protein binding sites with enhanced CLIP (eCLIP). Nat. Methods 13, 508–514 (2016).
Kladwang, W., Hum, J. & Das, R. Ultraviolet shadowing of RNA can cause significant chemical damage in seconds. Sci. Rep. 2, 517 (2012).
Hinman, M. N. & Lou, H. Diverse molecular functions of Hu proteins. Cell. Mol. Life Sci. 65, 3168–3181 (2008).
Noh, J. H. et al. HuR and GRSF1 modulate the nuclear export and mitochondrial localization of the lncRNA RMRP. Genes Dev. 30, 1224–1239 (2016).
Castello, A. et al. Comprehensive identification of RNA-binding domains in human cells. Mol. Cell 63, 696–710 (2016).
Ong, S.-E. et al. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell. Proteomics 1, 376–386 (2002).
Thul, P. J. et al. A subcellular map of the human proteome. Science 356, eaal3321 (2017).
Bose, A. et al. Glucose transporter recycling in response to insulin is facilitated by myosin Myo1c. Nature 420, 821–824 (2002).
Münnich, S., Taft, M. H. & Manstein, D. J. Crystal structure of human myosin 1c—the motor in GLUT4 exocytosis: implications for Ca2+ regulation and 14-3-3 binding. J. Mol. Biol. 426, 2070–2081 (2014).
Ihnatovych, I., Migocka-Patrzalek, M., Dukh, M. & Hofmann, W. A. Identification and characterization of a novel myosin Ic isoform that localizes to the nucleus. Cytoskeleton (Hoboken) 69, 555–565 (2012).
Qin, X. et al. Cocrystal structures of glycyl-tRNA synthetase in complex with tRNA suggest multiple conformational states in glycylation. J. Biol. Chem. 289, 20359–20369 (2014).
Shao, S., Brown, A., Santhanam, B. & Hegde, R. S. Structure and assembly pathway of the ribosome quality control complex. Mol. Cell 57, 433–444 (2015).
Yang, L., Yang, J., Huang, Y. & Liu, Z.-R. Phosphorylation of p68 RNA helicase regulates RNA binding by the C-terminal domain of the protein. Biochem. Biophys. Res. Commun. 314, 622–630 (2004).
Ranji, A., Shkriabai, N., Kvaratskhelia, M., Musier-Forsyth, K. & Boris-Lawrie, K. Features of double-stranded RNA-binding domains of RNA helicase A are necessary for selective recognition and translation of complex mRNAs. J. Biol. Chem. 286, 5328–5337 (2011).
White, M. R. et al. A dimer interface mutation in glyceraldehyde-3-phosphate dehydrogenase regulates its binding to AU-rich RNA. J. Biol. Chem. 290, 1770–1785 (2015).
Singh, R. & Green, M. Sequence-specific binding of transfer RNA by glyceraldehyde-3-phosphate dehydrogenase. Science 259, 365–368 (1993).
Harding, S. D. et al. The IUPHAR/BPS guide to pharmacology in 2018: updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Res. 46, D1091–D1106 (2018).
Buchan, J. R. & Parker, R. Eukaryotic stress granules: the ins and outs of translation. Mol. Cell 36, 932–941 (2009).
Kulic, I. M. et al. The role of microtubule movement in bidirectional organelle transport. Proc. Natl Acad. Sci. USA 105, 10011–10016 (2008).
Lin, C. et al. Active diffusion and microtubule-based transport oppose myosin forces to position organelles in cells. Nat. Commun. 7, 11814 (2016).
Athamneh, A. I. M. et al. Neurite elongation is highly correlated with bulk forward translocation of microtubules. Sci. Rep. 7, 7292 (2017).
Wang, C. et al. Dynamic tubulation of mitochondria drives mitochondrial network formation. Cell Res. 25, 1108–1120 (2015).
Karbowski, M. et al. Opposite effects of microtubule-stabilizing and microtubule-destabilizing drugs on biogenesis of mitochondria in mammalian cells. J. Cell Sci. 114, 281–291 (2001).
Kanehisa, M., Furumichi, M., Tanabe, M., Sato, Y. & Morishima, K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 45 (D1), D353–D361 (2017).
Hofmann, J. C., Husedzinovic, A. & Gruss, O. J. The function of spliceosome components in open mitosis. Nucleus 1, 447–459 (2010).
Tanenbaum, M. E., Stern-Ginossar, N., Weissman, J. S. & Vale, R. D. Regulation of mRNA translation during mitosis. Elife 4, e07957 (2015).
Shin, C. & Manley, J. L. The SR protein SRp38 represses splicing in M phase cells. Cell 111, 407–417 (2002).
Becher, I. et al. Pervasive protein thermal stability variation during the cell cycle. Cell 173, 1495–1507.e18 (2018).
Castello, A., Hentze, M. W. & Preiss, T. Metabolic enzymes enjoying new partnerships as RNA-binding proteins. Trends. Endocrinol. Metab. 26, 746–757 (2015).
Liao, Y. et al. The cardiomyocyte RNA-binding proteome: links to intermediary metabolism and heart disease. Cell Rep. 16, 1456–1469 (2016).
Anton, B. P. & Raleigh, E. A. Complete genome sequence of NEB 5-alpha, a derivative of Escherichia coli K-12 DH5α. Genome Announc. 4, e01245–e16 (2016).
Buskila, A. A., Kannaiah, S. & Amster-Choder, O. RNA localization in bacteria. RNA Biol. 11, 1051–1060 (2014).
Nevo-Dinur, K., Govindarajan, S. & Amster-Choder, O. Subcellular localization of RNA and proteins in prokaryotes. Trends Genet. 28, 314–322 (2012).
Plochowietz, A., Farrell, I., Smilansky, Z., Cooperman, B. S. & Kapanidis, A. N. In vivo single-RNA tracking shows that most tRNA diffuses freely in live bacteria. Nucleic Acids Res. 45, 926–937 (2017).
Smirnov, A. et al. Grad-seq guides the discovery of ProQ as a major small RNA-binding protein. Proc. Natl Acad. Sci. USA 113, 11591–11596 (2016).
Mercer, T. R. et al. Targeted sequencing for gene discovery and quantification using RNA CaptureSeq. Nat. Protoc. 9, 989–1009 (2014).
Wei, Z. et al. Coding and noncoding landscape of extracellular RNA released by human glioma stem cells. Nat. Commun. 8, 1145 (2017).
Batagov, A. O. & Kurochkin, I. V. Exosomes secreted by human cells transport largely mRNA fragments that are enriched in the 3′-untranslated regions. Biol. Direct. 8, 12 (2013).
Kanada, M. et al. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc. Natl Acad. Sci. USA 112, E1433–E1442 (2015).
Watanabe, A. et al. Raftlin is involved in the nucleocapture complex to induce poly(I:C)-mediated TLR3 activation. J. Biol. Chem. 286, 10702–10711 (2011).
Chang, C.-H. et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153, 1239–1251 (2013).
Garcin, E. D. GAPDH as a model non-canonical AU-rich RNA binding protein. Semin. Cell Dev. Biol. https://doi.org/10.1016/j.semcdb.2018.03.013 (2018).
White, M. R. & Garcin, E. D. The sweet side of RNA regulation: glyceraldehyde-3-phosphate dehydrogenase as a noncanonical RNA-binding protein. Wiley Interdiscip. Rev. RNA 7, 53–70 (2016).
Carmona, P., Rodríguez-Casado, A. & Molina, M. Conformational structure and binding mode of glyceraldehyde-3-phosphate dehydrogenase to tRNA studied by Raman and CD spectroscopy. Biochim. Biophys. Acta 1432, 222–233 (1999).
Taliaferro, J. M., Wang, E. T. & Burge, C. B. Genomic analysis of RNA localization. RNA Biol. 11, 1040–1050 (2014).
Moffitt, J. R., Pandey, S., Boettiger, A. N., Wang, S. & Zhuang, X. Spatial organization shapes the turnover of a bacterial transcriptome. Elife 5, e13065 (2016).
Huber, D. et al. SecA interacts with ribosomes in order to facilitate posttranslational translocation in bacteria. Mol. Cell 41, 343–353 (2011).
Wang, S., Yang, C.-I. & Shan, S.-O. SecA mediates cotranslational targeting and translocation of an inner membrane protein. J. Cell Biol. 216, 3639–3653 (2017).
van Teeffelen, S. et al. The bacterial actin MreB rotates, and rotation depends on cell-wall assembly. Proc. Natl Acad. Sci. USA 108, 15822–15827 (2011).
Rowlett, V. W. & Margolin, W. The bacterial Min system. Curr. Biol. 23, R553–R556 (2013).
Shih, Y.-L., Le, T. & Rothfield, L. Division site selection in Escherichia coli involves dynamic redistribution of Min proteins within coiled structures that extend between the two cell poles. Proc. Natl Acad. Sci. USA 100, 7865–7870 (2003).
Du, S. & Lutkenhaus, J. Assembly and activation of the Escherichia coli divisome. Mol. Microbiol. 105, 177–187 (2017).
Zerbino, D. R. et al. Ensembl 2018. Nucleic Acids Res. 46, D754–D761 (2018).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Patro, R., Duggal, G., Love, M. I., Irizarry, R. A. & Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419 (2017).
Sims, D. et al. CGAT: computational genomics analysis toolkit. Bioinformatics 30, 1290–1291 (2014).
Gobom, J., Nordhoff, E., Mirgorodskaya, E., Ekman, R. & Roepstorff, P. Sample purification and preparation technique based on nano-scale reversed-phase columns for the sensitive analysis of complex peptide mixtures by matrix-assisted laser desorption/ionization mass spectrometry. J. Mass. Spectrom. 34, 105–116 (1999).
Davis, S. et al. Expanding proteome coverage with charge ordered parallel ion analysis (CHOPIN) combined with broad specificity proteolysis. J. Proteome. Res. 16, 1288–1299 (2017).
McAlister, G. C. et al. MultiNotch MS3 enables accurate, sensitive, and multiplexed detection of differential expression across cancer cell line proteomes. Anal. Chem. 86, 7150–7158 (2014).
Dorfer, V. et al. MS Amanda, a universal identification algorithm optimized for high accuracy tandem mass spectra. J. Proteome. Res. 13, 3679–3684 (2014).
Gatto, L. & Lilley, K. S. MSnbase-an R/Bioconductor package for isobaric tagged mass spectrometry data visualization, processing and quantitation. Bioinformatics 28, 288–289 (2012).
Carlson, M. & Ortutay, C. UniProt.ws: R interface to UniProt web services. R package version 2.20.0. https://doi.org/10.18129/B9.bioc.UniProt.ws (2018).
Carlson, M. GO.db: a set of annotation maps describing the entire Gene Ontology. R package version 3.6.0. https://doi.org/10.18129/B9.bioc.GO.db (2018).
UniProt Consortium. UniProt: the universal protein knowledgebase. Nucleic Acids Res. 46, 2699 (2018).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer, New York, 2009).
Geiger, T., Wehner, A., Schaab, C., Cox, J. & Mann, M. Comparative proteomic analysis of eleven common cell lines reveals ubiquitous but varying expression of most proteins. Mol. Cell. Proteomics 11, M111.014050 (2012).
Wang, M., Herrmann, C. J., Simonovic, M., Szklarczyk, D. & von Mering, C. Version 4.0 of PaxDb: Protein abundance data, integrated across model organisms, tissues, and cell-lines. Proteomics 15, 3163–3168 (2015).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol. 57, 289–300 (1995).
Coimbatore Narayanan, B. et al. The Nucleic Acid Database: new features and capabilities. Nucleic Acids Res. 42, D114–D122 (2014).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–8,27–28 (1996).
Vizcaíno, J. A. et al. 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 44 (D1), D447–D456 (2016).
Acknowledgements
We would like to thank H. T. Parsons for donating E. coli cells, M. A. Elzek for culturing MCF10A cells, T. Mulroney for helping culturing U2OS cells and B. Fisher for kindly sharing equipment. E.V., T.S., R.M.L.Q., R.F.H., M.P. and M.R. are supported by Wellcome Trust, grant numbers 110170/Z/15/Z and 110071/Z/15/Z awarded to A.E.W. and K.S.L. V.D. is supported by Medical Research Council, grant number 5TR00. M.M.-S. is supported by a FEBS Long-Term Fellowship. G.H.T. and K.S.L. are supported by an IB Catalyst grant for Project DETOX (BB/N01040X/1).
Author information
Authors and Affiliations
Contributions
K.S.L., A.E.W., E.V., T.S. and R.M.L.Q. conceived the study. E.V., R.Q., T.S. and K.S.L. designed the experiments. E.V. optimized the initial OOPS protocol, prepared RNA-seq libraries and performed flow cytometry analysis. E.V. performed the SILAC, cellular subfractionation and nocodazole arrest experiments with assistance from R.M.L.Q. E. coli experiments were performed by M.M., E.V. and R.M.L.Q. U2OS RBP-capture was performed by M.P. and V.D. E.V. and R.M.L.Q. performed all additional experiments, including the RNA binding site experiment. R.M.L.Q performed all mass spectrometry. T.S. performed all data analysis, with the exception of the analysis of uridine content (D.-M.M.) and analysis of E. coli data (T.S., M.M.). E.V., T.S., R.M.L.Q. and K.S.L. interpreted results, with critical appraisal of findings from A.E.W., M.P., M.R., R.F.H. and V.D., including additional experiments (M.P., V.D. and R.F.H.). G.H.T. assisted with interpretation of E. coli data. M.M.-S. performed the protein–RNA structural analysis. T.S., E.V., R.M.L.Q., K.S.L. and M.M.-S. drafted the manuscript, with revision from A.E.W., R.F.H., G.H.T., D.-M.M., M.P. and M.R.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 OOPS unbiasedly recovers all PBR species.
(a) Absolute quantification of the total recovered RNA (PBR + Free RNA). Data shown as mean + /- SD of 3 independent experiments. (b) Representative Bioanalyzer representation of the RNAs size-profile obtained from the aqueous and interfaces. Red: CL (400mJ/cm2). Grey: NC. (c) Graphical representation of the RNA-origin analysed by RNA-seq. (d) Relative proportions of reads assigned to Ensembl gene biotypes for all analysed samples. (e) Correlation between two CL replicates (upper left panel) and a NC and 400 mJ/cm2 CL replicate (upper left panel), blue dashed line represents a 10-fold difference. Pearson correlation of all analysed samples with NC (right). (f) Correlation between the ratio of CL/NC RNA in the interface and the length of the transcript. (g) Percentage of windows deemed to be a site of protein binding. Windows split according to the mRNA feature type they overlap. Windows overlapping two none-intronic features classified as ambiguous. Random: random selection of the same number of windows, with the same distribution of read depths to obtain a null expectation based on read depth alone. (h) Relationship between uridine content in a window and the probability of the window being deemed to contain a protein binding site. (i) Read coverage across SNHG16 for CL (400 mJ/cm2) and NC replicates. Red boxes denote regions with consistently reduced coverage in CL.
Supplementary Figure 2 OOPS specifically recovers RBPs.
(a) The proportion of proteins missing in at least one CL replicate, at least one NC replicate, always missing in NC replicates or never missing in any sample. (b) Representative image of an acrylamide gel showing the interface protein content of a NC and CL samples. Left: coomassie-blue staining. Right: silver staining. (c) Crosslinking efficacy in HEK-293. Left panel: Relative proportions of free RNA (aqueous phase) and protein-bound RNA (RBR; interface) with increasing UV dosage. Right panel: Absolute quantification of the total recovered RNA (PBR + Free RNA). Data shown as mean + /- SD of 3 independent experiments. (d) Relationship between the number of peptides observed for a protein across all replicates and the p-value for CL-enrichment. Many proteins have insufficiently small p-value to pass 1% FDR threshold (dashed line) because of a lack of statistical power with few observations. (e) Classification of proteins as enriched or depleted in CL vs NC experiment. (f) Representative western blots for canonical RBPs (NCL (Nucleolin), PTB and HuR) and negative control proteins (H3 (Histone-H3) and β-tubulin). (g) The proportion of proteins which were missing in at least one RNAse replicate, at least one control replicate, always missing in the RNAse or control replicates or never missing. Int: Interface. Org: Organic. (h) Protein CL vs NC ratio and RNAse vs control ratio in the 3rd interface and 4th organic phases. (i) RNAse vs control ratio in the 3rd interface for GO annotated RBPs, other OOPS RBPs and glycoproteins. (j) GO terms over-represented in the proteins that migrate to the organic phase upon RNAse treatment.
Supplementary Figure 3 OOPS recovers known and new RBPs, even from under-represented cell compartments.
(a) Agreement between OOPS (n = 5) and Oligo(dT) RBP-Capture in HEK 293 cells. (b) Crosslinking efficacy in MCF10A. Upper panel: Relative proportions of free RNA (aqueous phase) and protein-bound RNA (RBR; interface) with increasing UV dosage. Lower panel: Absolute quantification of the total recovered RNA (PBR + Free RNA). Data shown as mean + /- SD of 3 independent experiments. (c) Biological process GO terms over-represented in tumoral-cell specific RBPs. (d) Detailed agreement between OOPS and published human RBPomes. (e) Overlap between the union of OOPS proteins identified in the 3 cell lines, the union of RICK and CARIC studies, and GO annotated RBPs. Proteins were restricted to those expressed in at least one of the three OOPS cell lines. (f-g) Top 10 Biological processes and Cellular compartment GO terms over-represented in the proteins identified in U2OS, HEK293 or MCF10A OOPS. (h) Top 10 Cellular Compartments GO terms over-represented in OOPS-exclusive RBPome.. (i) Representative western blot of the cellular subfractionation. Analysed markers are: nuclear fibrillarin (FBL), endoplasmic reticulum calreticulin (CALR), and cytosolic beta actin (ACTB), in heavy membranes (HM), light membranes (LM) and cytoskeleton and others (C/o) fractions.. (j) Normalised RBP abundance in the indicated fractions. Blue: transmembrane-containing RBPs. Grey: Non transmembrane-containing RBP.
Supplementary Figure 4 Direct assessment of cross-link sites validates most OOPS RBPs.
(a) Top: Schematic representation of the sequential digestion method used to identify the RNA-binding site. RNA-protein adducts are extracted from the interface and digested with Lys-C to yield RNA-peptides which are subsequently enriched by silica affinity column or ethanol precipitation. Enriched RNA-peptides are treated with RNAses followed by trypsin digestion. Peptides containing the UV-crosslinked nucleotide/RNA are retained by a TiO2 affinity column and the unbound fraction containing the peptide sequences adjacent to RNA crosslinking site is analysed by LC-MS/MS. Red = peptides containing site of crosslinking. Green = peptides adjacent to the RNA-binding site peptide. Bottom: Schematic representation of the process followed to determine crosslinking peptide. Blue squares represent a consistent binding site. Turquoise squares represent a promiscuous binding site.(b) Resolution and accuracy of RNA binding site detected is dependent on peptides detected. (c) Cryo-EM structure of the ribosome quality control complex (PDB ID 3J92). Proteins are shown as grey transparent surface, while RNA is depicted as transparent lime ribbon. Proteins previously detected as RBPs are highlighted in yellow, while the newly detected protein is shown in cyan. Zoomed in structures show this new protein as transparent cyan cartoon with the interacting residue detected by direct evidence shown as cyan sticks and RNA residues at 4 Å or less from it shown as lime sticks. (d-e) Crystal structures of (d) IMPDH2 in complex with ribavirin (PDB ID 1NF7) and (e) PARP1 in complex with rucaparib (PDB ID 1NF7). Proteins are shown as a cyan transparent cartoon and the protein region detected as RNA binding is shown in an opaque representation. Residues at 4 Å or less from inhibitor compounds (yellow sticks and surface representation) are shown as cyan sticks.
Supplementary Figure 5 Characterization of the nocodazole arrest experiments.
a) Top: Schematic representation of the nocodazole arrest/release experiment. Bottom-left: representative image of flow cytometry analysis of the cell-cycle stage by DNA content assessment. Bottom-right: Relative proportions of cells in G1, S and M phase for cells synchronised at each time-point is shown as the mean + /- SD of 3 independent experiments. (b) Top 10 Biological Processes and Cellular Compartment GO terms over-represented in the total proteome of synchronised cells versus 6 h post released cells. (c) Top 20 Biological Processes and Cellular Compartment GO terms over-represented in the RBPs with a significant increase in RNA-binding post-release in the nocodazole experiment. (d) Protein abundance for tRNA aminoacylation proteins with a significant increase in RNA-binding. Individual proteins with a significant increase are highlighted in green. (e) KEGG pathways over-represented in the RBPs with a significant decrease in RNA-binding post-release. (f) Protein abundance for splicing proteins with a significant increase in RNA-binding at 0 h. (g) Crosslink vs control and RNAse vs control protein abundance ratio in interface and organic phase for glycolytic and TCA cycle proteins (yellow).
Supplementary Figure 6 Characterization of the RBPome dynamics in the thymidine-nocodazole arrest experiments.
(a) Top-left: Schematic representation of the thymidine-nocodazole arrest/release experiment. Top-right: Relative proportions of cells in G1, S and G2/M phase for each time-point is shown as the mean + /- SD of 4 independent experiments Bottom: representative image of flow cytometry analysis of the cell-cycle stage by DNA content assessment. (b) Protein abundance from total proteome and OOPS extractions in thymidine-nocodazole arrested and released cells. Abundance z score normalised within each extraction type. Proteins hierarchically clustered across all samples as shown on left. (c) Top 10 Biological process and Cellular Compartment GO terms over-represented in the total proteome of thymidine-nocodazole arrested cells versus non treated cells. (d) Top 20 Biological process and Cellular Compartment GO terms over-represented in the RBPs with a significant increase in RNA-binding post-release in the thymidine-nocodazole experiment. (e) Correlation between p-values for KEGG pathway over-representation in the two nocodazole experiments. (f) Protein abundance for groups of overlapping KEGG pathways over-represented in proteins with a significant increase in RNA-binding. Individual proteins with a significant increase are highlighted in green. (g) Protein abundance for groups of overlapping KEGG pathways over-represented in proteins with a significant increase in RNA-binding. Individual proteins with a significant increase in RNA binding in 6 h vs 0 h are highlighted in green.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Note: Information about the deduplication of SENSE total RNA-seq, identification of protein binding sites, antibody details, uncropped western blots, SILAC labeling rationale, LC-MS/MS technical details, peptide-to-protein assignment and identification of RNA binding sites
Supplementary Table 1
Verification of the OOPS method
Supplementary Table 2
RBPs identified by OOPS and oligo(dT) RBP-capture
Supplementary Table 3
Subcellular OOPS
Supplementary Table 4
RNA binding sites
Supplementary Table 5
RNA binding changes identified in nocodazole and thymidine nocodazole experiments
Supplementary Table 6
MS parameters and TMT labeling
Rights and permissions
About this article
Cite this article
Queiroz, R.M.L., Smith, T., Villanueva, E. et al. Comprehensive identification of RNA–protein interactions in any organism using orthogonal organic phase separation (OOPS). Nat Biotechnol 37, 169–178 (2019). https://doi.org/10.1038/s41587-018-0001-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41587-018-0001-2