Abstract
Ischaemic diseases such as critical limb ischaemia and myocardial infarction affect millions of people worldwide1. Transplanting endothelial cells (ECs) is a promising therapy in vascular medicine, but engrafting ECs typically necessitates co-transplanting perivascular supporting cells such as mesenchymal stromal cells (MSCs), which makes clinical implementation complicated2,3. The mechanisms that enable MSCs to facilitate EC engraftment remain elusive. Here we show that, under cellular stress, MSCs transfer mitochondria to ECs through tunnelling nanotubes, and that blocking this transfer impairs EC engraftment. We devised a strategy to artificially transplant mitochondria, transiently enhancing EC bioenergetics and enabling them to form functional vessels in ischaemic tissues without the support of MSCs. Notably, exogenous mitochondria did not integrate into the endogenous EC mitochondrial pool, but triggered mitophagy after internalization. Transplanted mitochondria co-localized with autophagosomes, and ablation of the PINK1–Parkin pathway negated the enhanced engraftment ability of ECs. Our findings reveal a mechanism that underlies the effects of mitochondrial transfer between mesenchymal and endothelial cells, and offer potential for a new approach for vascular cell therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data supporting the findings of this study are available in the paper and its Supplementary Information. The RNA-seq datasets generated during the study are available in the Gene Expression Omnibus repository, under the accession number GSE255798. Source data are provided with this paper.
References
Nowbar, A. N., Gitto, M., Howard, J. P., Francis, D. P. & Al-Lamee, R. Mortality from ischemic heart disease. Circ. Cardiovasc. Qual. Outcomes 12, e005375 (2019).
Loffredo, F. & Lee, R. T. Therapeutic vasculogenesis. Circ. Res. 103, 128–130 (2008).
Melero-Martin, J. M. et al. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ. Res. 103, 194–202 (2008).
Beckman, J. A., Schneider, P. A. & Conte, M. S. Advances in revascularization for peripheral artery disease: revascularization in PAD. Circ. Res. 128, 1885–1912 (2021).
Carmeliet, P. & Jain, R. K. Molecular mechanisms and clinical applications of angiogenesis. Nature 473, 298 (2011).
Cooke, J. P. & Losordo, D. W. Modulating the vascular response to limb ischemia. Circ. Res. 116, 1561–1578 (2015).
Wang, K., Lin, R.-Z. & Melero-Martin, J. M. Bioengineering human vascular networks: trends and directions in endothelial and perivascular cell sources. Cell. Mol. Life Sci. 76, 421–439 (2019).
Islam, M. N. et al. Mitochondrial transfer from bone-marrow–derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 18, 759 (2012).
Hayakawa, K. et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535, 551 (2016).
Jain, R. K. Molecular regulation of vessel maturation. Nat. Med. 9, 685–693 (2003).
Andrae, J., Gallini, R. & Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Gene Dev. 22, 1276–1312 (2008).
Rustom, A., Saffrich, R., Markovic, I., Walther, P. & Gerdes, H.-H. Nanotubular highways for intercellular organelle transport. Science 303, 1007–1010 (2004).
Zhang, Y. et al. iPSC-MSCs with high intrinsic MIRO1 and sensitivity to TNF-α yield efficacious mitochondrial transfer to rescue anthracycline-induced cardiomyopathy. Stem Cell Rep. 7, 749–763 (2016).
Hase, K. et al. M-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex. Nat. Cell Biol. 11, 1427–1432 (2009).
Kitani, T., Kami, D., Matoba, S. & Gojo, S. Internalization of isolated functional mitochondria: involvement of macropinocytosis. J. Cell. Mol. Med. 18, 1694–1703 (2014).
Youle, R. J. & Narendra, D. P. Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 12, 9–14 (2011).
Jin, S. M. & Youle, R. J. PINK1- and Parkin-mediated mitophagy at a glance. J. Cell Sci. 125, 795–799 (2012).
Liu, K. et al. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia–reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc. Res. 92, 10–18 (2014).
Liang, X. et al. Direct administration of mesenchymal stem cell‐derived mitochondria improves cardiac function after infarction via ameliorating endothelial senescence. Bioeng. Transl. Med. 8, e10365 (2023).
Borcherding, N. et al. Dietary lipids inhibit mitochondria transfer to macrophages to divert adipocyte-derived mitochondria into the blood. Cell Metab. 34, 1499–1513 (2022).
Kami, D. & Gojo, S. From cell entry to engraftment of exogenous mitochondria. Int. J. Mol. Sci. 21, 4995 (2020).
Elliott, R. L., Jiang, X. P. & Head, J. F. Mitochondria organelle transplantation: introduction of normal epithelial mitochondria into human cancer cells inhibits proliferation and increases drug sensitivity. Breast Cancer Res. Treat. 136, 347–354 (2012).
Chang, J.-C. et al. Allogeneic/xenogeneic transplantation of peptide-labeled mitochondria in Parkinson’s disease: restoration of mitochondria functions and attenuation of 6-hydroxydopamine–induced neurotoxicity. Transl. Res. 170, 40–56 (2016).
Kaza, A. K. et al. Myocardial rescue with autologous mitochondrial transplantation in a porcine model of ischemia/reperfusion. J. Thorac. Cardiovasc. Surg. 153, 934–943 (2017).
Emani, S. M., Piekarski, B. L., Harrild, D., Del Nido, P. J. & McCully, J. D. Autologous mitochondrial transplantation for dysfunction after ischemia-reperfusion injury. J. Thorac. Cardiovasc. Surg. 154, 286–289 (2017).
Bertero, E., Maack, C. & O’Rourke, B. Mitochondrial transplantation in humans: “magical” cure or cause for concern? J. Clin. Invest. 128, 5191–5194 (2018).
Lightowlers, R. N., Chrzanowska‐Lightowlers, Z. M. & Russell, O. M. Mitochondrial transplantation—a possible therapeutic for mitochondrial dysfunction? EMBO Rep. 21, e50964 (2020).
Ashrafi, G. & Schwarz, T. L. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 20, 31–42 (2013).
Moreau, K., Luo, S. & Rubinsztein, D. C. Cytoprotective roles for autophagy. Curr. Opin. Cell Biol. 22, 206–211 (2010).
Gao, Y. et al. Role of Parkin-mediated mitophagy in the protective effect of polydatin in sepsis-induced acute kidney injury. J. Transl. Med. 18, 114 (2020).
Livingston, M. J. et al. Clearance of damaged mitochondria via mitophagy is important to the protective effect of ischemic preconditioning in kidneys. Autophagy 15, 2142–2162 (2019).
Sun, Z. et al. MSC-derived extracellular vesicles activate mitophagy to alleviate renal ischemia/reperfusion injury via the miR-223-3p/NLRP3 axis. Stem Cells Int. 2022, 6852661 (2022).
Mahrouf-Yorgov, M. et al. Mesenchymal stem cells sense mitochondria released from damaged cells as danger signals to activate their rescue properties. Cell Death Differ. 24, 1224–1238 (2017).
Zhu, W. et al. Mesenchymal stem cells ameliorate hyperglycemia-induced endothelial injury through modulation of mitophagy. Cell Death Dis. 9, 837 (2018).
Kim, M. J., Hwang, J. W., Yun, C.-K., Lee, Y. & Choi, Y.-S. Delivery of exogenous mitochondria via centrifugation enhances cellular metabolic function. Sci. Rep. 8, 3330 (2018).
Melero-Martin, J. M. et al. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood 109, 4761–4768 (2007).
Lin, R.-Z. et al. Human endothelial colony-forming cells serve as trophic mediators for mesenchymal stem cell engraftment via paracrine signaling. Proc. Natl Acad. Sci. USA 111, 10137–10142 (2014).
Acknowledgements
Illustrations were partially created with BioRender.com. This work was supported by a grant from the National Institutes of Health (NIH) (R01HL152133 to J.M.M.-M.). Work in the N.P. laboratory was supported by a NIH NIAMS R01 grant.
Author information
Authors and Affiliations
Contributions
R.-Z.L. and J.M.M.-M. conceived and designed the project. R.-Z.L., G.-B.I., A.C.L., Y.Z., X.H., J.N. and H.-W.T. performed the experimental work. All authors discussed and analysed the data and edited the results. R.-Z.L. and J.M.M.-M. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Jonathan Brestoff, Anna Randi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
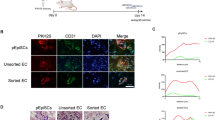
Extended Data Fig. 1 Stromal cell support is essential for human EC engraftment.
Grafts comprising human ECs with or without MSCs were subcutaneously implanted in immunodeficient nude mice. a, H&E staining of 7-day explants with human ECFCs, HUVECs, and wat-ECs, highlighting perfused vessels (yellow arrowheads). Insets show day 7 macroscopic views. Scale bar, 100 μm. b, Microvessel density at day 7 with various human EC types; **P ≤ 0.01, ***P ≤ 0.001 (n = 3; unpaired t-test). c, Bioluminescence of lucif-EC grafts with or without MSCs. d, Time-dependent quantification of bioluminescence; *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 (n = 3; unpaired t-test). e, EC apoptosis assessed by flow cytometry at 24 h post-implantation with/without MSCs, MSC-CM, VEGF, bFGF, and AG1296; *P ≤ 0.05, ***P ≤ 0.001 (n = 3; unpaired t-test). f, Proteomic dot blotting reveals unique pro-angiogenic factors in MSC-conditioned media. g, H&E staining of 7-day explants with multiple conditions; yellow arrowheads indicate perfused vessels. Scale bar, 100 μm. h, Day-7 microvessel density; ***P ≤ 0.001 (n = 3; unpaired t-test). All data are mean ± s.e.m. n are biological replicates (e) and independent animals (b,d,h).
Extended Data Fig. 2 Formation of mitochondria-laden TNTs by MSCs in the presence of ECs.
a, Schematic of 24 h co-culture of mitoRed-MSCs with human ECs. DsRed+ mitochondria in TNTs visualized via fluorescence microscopy. Scale bar, 40 μm. b, Immunofluorescence reveals F-actin and microtubule components in TNTs. Scale bar, 5 μm. c, Flow cytometry gating strategy to evaluate mitochondrial transfer and eliminate doublets and aggregates. d, Proportion of ECs (CD31+) with mitoDsRed+ mitochondria in 1:1 co-culture, indicating plated cell number. e, Proportion of mitoDsRed+ mitochondria-receiving ECs at varying MSC:EC ratios, with constant total cell density (4 × 105 cells). f, Comparison of mitochondrial transfer in 2D vs. 3D culture and under normoxic vs. hypoxic conditions (4 × 106 cells). g, mitoRed-ECs cultured with/without MSCs show negligible DsRed+ mitochondria-laden TNT formation, even after TNF treatment. F-actin visualized by FITC-phalloidin. Scale bars, 10 μm. h, Fluorescent quantification confirms minimal TNT formation by ECs under tested conditions (n = 3). i, Flow cytometry quantification of DsRed+ mitochondria in MSCs (CD31−) indicates minimal transfer (~1%) from mitoRed-ECs (n = 3). j, Proportion of ECs (CD31+ ) with mitoDsRed+ mitochondria in 1:1 (ECs + mitoRed-MSCs) co-culture in the presence of angiogenic factors VEGF (10 ng/mL) or bFGF (10 ng/mL) (n = 3-4; one-way ANOVA followed by Bonferroni’s post-test). All data are mean ± s.e.m. n are biological replicates (h,i,j).
Extended Data Fig. 3 Regulation of TNT formation and mitochondrial transfer from MSCs to ECs.
a, Human ECs cultured in 2D plates or 3D hydrogel had conditioned media analysed for cytokine secretion using proteomic dot blotting arrays; selected cytokines predominantly secreted in 3D are marked. b, Blot intensities quantified by ImageJ. c, Effect of IL-1α and IL-1β on TNT formation in MSCs. MSCs were exposed to IL-1α, IL-1β, or an anti-IL-1α antibody, and changes in TNT formation were observed and quantified at 24 h. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 (n = 17, MSCs; n = 10, +IL-1β; n = 6, +IL-1α; n = 11, +ECs; n = 11, +ECs + anti-IL-1α; one-way ANOVA followed by Bonferroni’s post-test). d, shRNA silencing of TNFAIP2 in mitoRed-MSCs confirmed by qPCR against GAPDH, *P ≤ 0.05 (n = 2; unpaired two-tailed t-test). e, Reduced DsRed+ TNTs in shTNFAIP2-MSCs compared to controls; scale bar, 10 μm. f, MIRO1 silencing in mitoRed-MSCs confirmed by qPCR, *P ≤ 0.05 (n = 2; unpaired two-tailed t-test). g, F-actin in mitoRed-MSCs visualized by FITC-phalloidin; scale bar, 10 μm. h, Lower DsRed+ mitochondria count per TNT in shMIRO1-MSCs, ***P ≤ 0.001 (n = 30 TNTs; unpaired two-tailed t-test). i, Co-cultures of ECs and mitoRed-MSCs assessed by flow cytometry revealed reduced mitochondrial transfer from shMIRO1-MSCs compared to control MSCs, ***P ≤ 0.001 (n = 4; unpaired two-tailed t-test). All data are mean ± s.e.m. n are independent fields (c,h) and biological replicates (d,f,i,j).
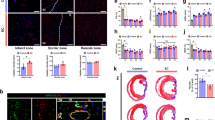
Extended Data Fig. 4 In vivo temporal dynamics of mitochondrial transfer from MSCs to ECs.
a, Depiction of mitoRed-MSCs containing DsRed+ mitochondria and subcutaneously co-transplanted with human ECs into immunodeficient nude mice. b, Day-7 post-transplant, immunofluorescence of explanted grafts stained with UEA1 lectin showcases human ECs. Red fluorescence indicates DsRed+ mitochondria, identified with white arrows. UEA1+ ECs containing DsRed+ mitochondria are marked with a yellow arrowhead. Control grafts with unlabelled MSCs, showing no DsRed signal, are displayed on the left. Blood vessel lumens are asterisked. Scale bars, 50 μm. c, Different time-point immunofluorescent images of explants highlight DsRed+ mitochondria in UEA1+ ECs (yellow arrowhead) on day 7, absent on day 14. However, administering TNF (contrary to saline) on day 14 reinstated the DsRed+ mitochondrial transfer into UEA1+ ECs, seen on day 16. Scale bars, 50 μm. d, Diagram representing the time course appearance of DsRed+ mitochondria (mitoRed) in the grafted ECs.
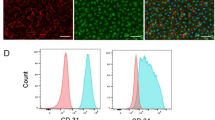
Extended Data Fig. 5 Artificial transplantation of exogenous mitochondria into human ECs.
a, Phase contrast images combined with red fluorescence show cultured ECs 4 h post artificial transplantation with DsRed+ mitochondria (mitoAT-ECs); Scale bar, 200 μm. b, Flow cytometry highlights a significant proportion of DsRed+ mitoAT-ECs. c, Confocal imaging reveals DsRed+ mitochondria inside mitoAT-ECs; Scale bar, 10 μm. d, Depiction of the pPB-mitoAPEX2 piggyBac vector carrying APEX2 and mitochondria-targeting sequences. APEX2, an enhanced soybean ascorbate peroxidase, acts as a tag for TEM. e, Method to visualize APEX2+ mitochondria using TEM: APEX2-expressing cells are fixed, then treated with diaminobenzidine (DAB) and hydrogen peroxide. APEX2 catalyses the DAB polymerization, generating TEM contrast after osmium treatment. f, TEM contrasts APEX2-labelled mitochondria in mitoAPEX2-transfected cells with unlabelled counterparts in controls; Scale bar, 500 nm. g, High-magnification TEM of an APEX2-labelled mitochondrion. h, Mitochondria from mitoAPEX2-MSCs transplanted into ECs show APEX2+ mitochondria in mitoAT-ECs at 4 h; Scale bar, 500 nm.
Extended Data Fig. 6 Enhanced mitochondrial respiration, apoptosis resistance and migration capacity in mitoAT-ECs.
a, Schematic of OCR profile (Seahorse analysis) with specific mitochondrial respiration parameters. Abbreviations: FCCP, carbonyl cyanide-p-trifluoromethoxyphenyl hydrazone. b, Basal respiration, maximal respiration, spare respiratory capacity, proton leak, and non-mitochondrial respiration in ECs and mitoAT-ECs. ***P ≤ 0.001 (n = 6; unpaired two-tailed t-test). c, ATP production in mitoAT-ECs measured at 1 and 7 days after a one-time artificial mitochondrial transplantation. ***P ≤ 0.001 (n = 6 at day 1, n = 10 at day 7; unpaired two-tailed t-test). d, Post-mitochondrial transplantation, mitoAT-ECs exposed to 200 µM H2O2 for 12 h were analysed for apoptosis using flow cytometry (PI/Annexin-V staining), with non-transplanted ECs as controls. e, Percentage of apoptotic (Annexin-V+) ECs post H2O2 exposure. **P ≤ 0.01, ***P ≤ 0.001 (n = 3-4; one-way ANOVA with Bonferroni’s post-test). f, A standard wound closure assay depicts migration capacity, with wound areas at 0 h and 12 h in mitoAT-ECs compared to control ECs. g, Quantification of wound gap closure in mitoAT-ECs transplanted with mitochondria from various donors, indicating improved closure rates, ***P ≤ 0.001 (n = 3; one-way ANOVA followed by Bonferroni’s post-test). h, Relative ATP production levels in ECs measured 24 h after receiving different concentrations of mitochondria (mitoAT-ECs). ECs without mitochondrial transfer served as control. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 compared to control (n = 3–7; unpaired two-tailed t-test). i, Comparison of ATP production in ECs transplanted with exogenous mitochondria (mitoAT-ECs) or lysosomes (lysoAT-ECs). **P ≤ 0.01, ***P ≤ 0.001 (n = 10; one-way ANOVA with Bonferroni’s post-test). j, Histological evaluations of subcutaneous grafts with lysoAT-ECs at 7 days post-transplantation. Scale bar, 100 μm. Quantitative analysis of microvascular density displaying reduced vascularization potential (n = 4). All data are mean ± s.e.m. n are biological replicates (b,c,e,g,h,i) and independent animals (j).
Extended Data Fig. 7 Temporal analysis of mtDNA heteroplasmy in mitoAT-ECs after mitochondrial transplantation.
a, mtDNA sequencing showcases four specific loci with heteroplasmy in hypervariable regions, highlighting a single nucleotide difference between ECs and MSCs. Depicted sequences represent ECs (monoculture), MSCs (monoculture), ECs + MSCs (1:1 co-culture), and mitoAT-ECs (monoculture) for the chosen loci. b, Summary table detailing heteroplasmy at four loci in the hypervariable mtDNA regions across ECs and MSCs.
Extended Data Fig. 8 Quantitative flow cytometry analysis of exogenous mitochondrial integration using the split-GFP system.
a, Schematic of the split-GFP system, with GFP-1–10 in EC mitochondria and GFP-11 in mitoRed-MSCs. Fluorescence signals fusion between MSC-derived and EC mitochondria. b, Flow cytometry gating strategy for mitochondria, using size-specific microbeads as reference. c, Flow cytometry analysis of isolated mitochondria illustrating the proportion of non-fused DsRed+ (red box) and fused DsRed+GFP+ (yellow box) mitochondria across experimental groups. d, Mitochondrial integration post-transplantation analysis. Proportions of DsRed+ (red box) and DsRed+GFP+ (yellow box) in mitoAT-EC-derived mitochondria assessed at 24 h post-transplantation, with EC-derived mitochondria as the negative control. Representative flow cytometry data showing a dose-dependent increase in DsRed+ mitochondria at 24 h with double (×2) and quadruple (×4) transplanted mitochondria, consistently <0.1% GFP+.
Extended Data Fig. 9 Transplantation of mtDNA-free mitochondria enhances ATP in ECs.
a, Diagram illustrating the engineering of mtDNA-free cells (mitoRed-ρ0) using a Dox-regulated MTS-EcoRI-GFP complex for mtDNA degradation. b, PCR gel confirms the thorough depletion of mtDNA in the mitoRed-ρ0-293T cell line after a 48-hour Dox treatment. c, Transplantation of mtDNA-free mitochondria from mitoRed-ρ0-MSCs into ECs. Quantification of ATP production in both ECs and mitoAT-ECs. *P ≤ 0.05, ***P ≤ 0.001 (n = 3; one-way ANOVA with Bonferroni’s post-test). d, Transplantation of normal and mtDNA-free mitochondria into ρ0-ECs. ATP production in ρ0-ECs measured before and 24 h after exogenous mitochondrial transplantation. ***P ≤ 0.001 (n = 4, ECs; n = 8, ρ0-ECs; n = 8, mitoAT-ECs with normal mito; n = 6, mitoAT-ECs with mtDNA-free mito; one-way ANOVA followed by Bonferroni’s post-test). All data are mean ± s.e.m. n are biological replicates (c,d).
Extended Data Fig. 10 Exogenous mitochondrial transplantation induces mitophagy in mitoAT-ECs.
a, Analysis of autophagic flux in mitoAT-ECs, showing LC3B-I to LC3B-II conversion with Bafilomycin A1. b, Flow cytometry analysis showing PINK1 presence on isolated mitochondria from control MSCs (shCTR-MSCs) but not from shPINK1-MSCs. Quantification of the percentage of PINK1-positive in isolated mitochondria from control versus shPINK1-MSCs. ***P ≤ 0.001 (n = 4; unpaired two-tailed t-test). c, Western blot analysis demonstrating the presence of PINK1 (63 kDa) in lysates from both MSCs and isolated mitochondria (red line box), with TOM20 (16 kDa) as a mitochondrial marker and GAPDH (36 kDa) as a cytosolic control. d, Immunofluorescence indicates co-localization of exogenous DsRed+ mitochondria (Red) with endogenous Parkin (Alexa 647) in mitoAT-ECs 24 h post-transplantation (white arrows). Scale bar, 100 μm; insets #1–4, 10 µm. e–h, Evaluation of MSC viability and functionality after PINK1 silencing (shRNA). e, Morphological observations of MSCs with shRNA against PINK1 (shPINK1-MSCs) vs. control shRNA (shCTR-MSCs) show standard mesenchymal cell morphology. Scale bar, 100 μm. f, Flow cytometry using PI/Annexin-V highlights the high viability of shPINK1-MSCs after lentiviral transduction (n = 4). g, qPCR analysis measures angiogenic growth factor expression (ANGPT, CXCL12, VEGF) in shCTR-MSCs vs. shPINK1-MSCs (n = 4; unpaired two-tailed t-test). h, In vitro assay of EC vascular network formation, using conditioned medium (CM) from shPINK1-MSCs vs. shCTR-MSCs (n = 4; unpaired two-tailed t-test). Scale bar, 200 μm. i, Immunofluorescence detection of LC3B+ autophagosomes in mitoAT-ECs. Effects of Parkin and PINK1 silencing (shRNA) in either the donor MSC mitochondria (mito) or recipient ECs. DAPI denotes cell nuclei. Scale bar, 10 μm. All data are mean ± s.e.m. n are biological replicates (b,f,g,h). For gel source data, see Supplementary Fig. 1.
Supplementary information
Supplementary Figure 1
Uncropped immunoblots from main Figure 4 and Extended Data Figure 10.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, RZ., Im, GB., Luo, A.C. et al. Mitochondrial transfer mediates endothelial cell engraftment through mitophagy. Nature 629, 660–668 (2024). https://doi.org/10.1038/s41586-024-07340-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07340-0
This article is cited by
-
Current Advances and Challenges in Stem Cell-Based Regenerative Therapy for Chronic Limb-Threatening Ischemia
Current Treatment Options in Cardiovascular Medicine (2025)
-
Mitochondria transfer-based therapies reduce the morbidity and mortality of Leigh syndrome
Nature Metabolism (2024)
-
Pharmacokinetic characteristics of mesenchymal stem cells in translational challenges
Signal Transduction and Targeted Therapy (2024)
-
Artificially transplanted mitochondria in endothelial cells promote mitophagy
Nature Reviews Cardiology (2024)
-
Cells destroy donated mitochondria to build blood vessels
Nature (2024)