Abstract
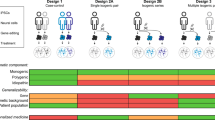
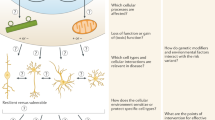
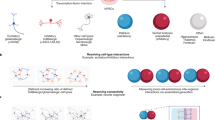
Human induced pluripotent stem cells (hiPSCs) were first generated in 2007, but the full translational potential of this valuable tool has yet to be realized. The potential applications of hiPSCs are especially relevant to neurology, as brain cells from patients are rarely available for research. hiPSCs from individuals with neuropsychiatric or neurodegenerative diseases have facilitated biological and multi-omics studies as well as large-scale screening of chemical libraries. However, researchers are struggling to improve the scalability, reproducibility and quality of this descriptive disease modelling. Addressing these limitations will be the first step towards a new era in hiPSC research — that of predictive disease modelling — involving the correlation and integration of in vitro experimental data with longitudinal clinical data. This approach is a key element of the emerging precision medicine paradigm, in which hiPSCs could become a powerful diagnostic and prognostic tool. Here, we consider the steps necessary to achieve predictive modelling of neurodegenerative disease with hiPSCs, using Huntington disease as an example.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007).
Shi, Y., Inoue, H., Wu, J. C. & Yamanaka, S. Induced pluripotent stem cell technology: a decade of progress. Nat. Rev. Drug Discov. 16, 115–130 (2017).
Rubin, L. L. Stem cells and drug discovery: the beginning of a new era? Cell 132, 549–552 (2008).
Trevisan, M. et al. Modeling viral infectious diseases and development of antiviral therapies using human induced pluripotent stem cell-derived systems. Viruses 7, 3835–3856 (2015).
Huang, J. et al. SARS-CoV-2 infection of pluripotent stem cell-derived human lung alveolar type 2 cells elicits a rapid epithelial-intrinsic inflammatory response. Cell Stem Cell 27, 962–973.e7 (2020).
Cayo, M. A. et al. A drug screen using human iPSC-derived hepatocyte-like cells reveals cardiac glycosides as a potential treatment for hypercholesterolemia. Cell Stem Cell 20, 478–489.e5 (2017).
Eglen, R. M. & Reisine, T. Human iPS cell-derived patient tissues and 3D cell culture part 1: target identification and lead optimization. SLAS Technol. 24, 3–17 (2019).
Kandasamy, K. et al. Prediction of drug-induced nephrotoxicity and injury mechanisms with human induced pluripotent stem cell-derived cells and machine learning methods. Sci. Rep. 5, 12337 (2015).
Wu, Y.-Y., Chiu, F.-L., Yeh, C.-S. & Kuo, H.-C. Opportunities and challenges for the use of induced pluripotent stem cells in modelling neurodegenerative disease. Open Biol. 9, 180177 (2019).
Li, L., Chao, J. & Shi, Y. Modeling neurological diseases using iPSC-derived neural cells: iPSC modeling of neurological diseases. Cell Tissue Res. 371, 143–151 (2018).
McNeish, J., Gardner, J. P., Wainger, B. J., Woolf, C. J. & Eggan, K. From dish to bedside: lessons learned while translating findings from a stem cell model of disease to a clinical trial. Cell Stem Cell 17, 8–10 (2015).
Okano, H., Yasuda, D., Fujimori, K., Morimoto, S. & Takahashi, S. Ropinirole, a new ALS drug candidate developed using iPSCs. Trends Pharmacol. Sci. 41, 99–109 (2020).
Kovalchuk, M. O. et al. Acute effects of riluzole and retigabine on axonal excitability in patients with amyotrophic lateral sclerosis: a randomized, double-blind, placebo-controlled, crossover trial. Clin. Pharmacol. Ther. 104, 1136–1145 (2018).
Yousefi, N., Abdollahii, S., Kouhbanani, M. A. J. & Hassanzadeh, A. Induced pluripotent stem cells (iPSCs) as game-changing tools in the treatment of neurodegenerative disease: mirage or reality? J. Cell. Physiol. 235, 9166–9184 (2020).
Ahmadian-Moghadam, H., Sadat-Shirazi, M. S. & Zarrindast, M. R. Therapeutic potential of stem cells for treatment of neurodegenerative diseases. Biotechnol. Lett. 42, 1073–1101 (2020).
Levy, M., Boulis, N., Rao, M. & Svendsen, C. N. Regenerative cellular therapies for neurologic diseases. Brain Res. 1638, 88–96 (2016).
Allsopp, T. E., Ebneth, A. & Cabrera-Socorro, A. Deploying human pluripotent stem cells to treat central nervous system disorders: facts, challenges and realising the potential. Stem Cell Res. 41, 101581 (2019).
Schweitzer, J. S. et al. Personalized iPSC-derived dopamine progenitor cells for Parkinson’s disease. N. Engl. J. Med. 382, 1926–1932 (2020).
Guhr, A. et al. Recent trends in research with human pluripotent stem cells: impact of research and use of cell lines in experimental research and clinical trials. Stem Cell Rep. 11, 485–496 (2018).
Marton, R. M. & Ioannidis, J. P. A. A comprehensive analysis of protocols for deriving dopaminergic neurons from human pluripotent stem cells. Stem Cell Transl. Med. 8, 366–374 (2019).
Chambers, S. M. et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 27, 275–280 (2009).
Kriks, S. et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 480, 547–551 (2011).
Hu, B.-Y. et al. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc. Natl Acad. Sci. USA 107, 4335–4340 (2010).
Yaffe, M. P., Noggle, S. A. & Solomon, S. L. Raising the standards of stem cell line quality. Nat. Cell Biol. 18, 236–237 (2016).
Kim, K. et al. Epigenetic memory in induced pluripotent stem cells. Nature 467, 285–290 (2010).
Vaskova, E. A., Stekleneva, A. E., Medvedev, S. P. & Zakian, S. M. ‘Epigenetic memory’ phenomenon in induced pluripotent stem cells. Acta Naturae 5, 15–21 (2013).
Lo Sardo, V. et al. Influence of donor age on induced pluripotent stem cells. Nat. Biotechnol. 35, 69–74 (2017).
Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 14, R115 (2013).
Huh, C. J. et al. Maintenance of age in human neurons generated by microRNA-based neuronal conversion of fibroblasts. eLife 5, e18648 (2016).
Kang, E. et al. Age-related accumulation of somatic mitochondrial DNA mutations in adult-derived human iPSCs. Cell Stem Cell 18, 625–636 (2016).
Kirkeby, A. et al. Predictive markers guide differentiation to improve graft outcome in clinical translation of hESC-based therapy for Parkinson’s disease. Cell Stem Cell 20, 135–148 (2017).
Kee, N. et al. Single-cell analysis reveals a close relationship between differentiating dopamine and subthalamic nucleus neuronal lineages. Cell Stem Cell 20, 29–40 (2017).
Daley, G. Q. et al. Setting global standards for stem cell research and clinical translation: the 2016 ISSCR guidelines. Stem Cell Rep. 6, 787–797 (2016).
International Society for Stem Cell Research. Guidelines for Stem Cell Research and Clinical Translation (ISSCR, 2016).
De Sousa, P. A. et al. Rapid establishment of the European Bank for induced Pluripotent Stem Cells (EBiSC) – the Hot Start experience. Stem Cell Res. 20, 105–114 (2017).
Huang, C. Y. et al. Human iPSC banking: barriers and opportunities. J. Biomed. Sci. 26, 87 (2019).
Harvey, E., Hewison, C., Nevalainen, D. E. & Lloyd, H. L. Maintaining quality in blood banking. Blood Rev. 9, 15–24 (1995).
Toombs, J. et al. Generation of twenty four induced pluripotent stem cell lines from twenty four members of the Lothian Birth Cohort 1936. Stem Cell Res. 46, 101851 (2020).
Sarvari, M., Alavi-Moghadam, S., Larijani, B., Rezazadeh, I. & Arjmand, B. in Biomedical Product Development: Bench to Bedside, Ch 6 (eds Arjmand, B., Payab, M. & Goodarzi, P.) 61–68 (Springer, 2020).
Barker, R. A., Parmar, M., Studer, L. & Takahashi, J. Human trials of stem cell-derived dopamine neurons for Parkinson’s disease: dawn of a new era. Cell Stem Cell 21, 569–573 (2017).
Lee, K. M. et al. The application of human pluripotent stem cells to model the neuronal and glial components of neurodevelopmental disorders. Mol. Psychiatry 25, 368–378 (2020).
Ardhanareeswaran, K., Mariani, J., Coppola, G., Abyzov, A. & Vaccarino, F. M. Normal development of neuronal networks requires a delicate balance of proliferation and differentiation of specific neuronal lineages, and appropriate migration and integration of these specific neuronal subtypes into neuronal circuits. Nat.Rev. Neurol. 13, 265–278 (2017).
Wang, M., Zhang, L. & Gage, F. H. Modeling neuropsychiatric disorders using human induced pluripotent stem cells. Protein Cell 11, 45–59 (2020).
Noh, H., Shao, Z., Coyle, J. T. & Chung, S. Modeling schizophrenia pathogenesis using patient-derived induced pluripotent stem cells (iPSCs). Biochim. Biophys. Acta Mol. Basis Dis. 1863, 2382–2387 (2017).
Csobonyeiova, M., Polak, S. & Danisovic, L. Recent overview of the use of iPSCs Huntington’s disease modeling and therapy. Int. J. Mol. Sci. 21, 2239 (2020).
Han, F. et al. The application of patient-derived induced pluripotent stem cells for modeling and treatment of Alzheimer’s disease. Brain Sci. Adv. 5, 21–40 (2019).
Majolo, F., Marinowic, D. R., MacHado, D. C. & Da Costa, J. C. Important advances in Alzheimer’s disease from the use of induced pluripotent stem cells. J. Biomed. Sci. 26, 15 (2019).
Ke, M., Chong, C. M. & Su, H. Using induced pluripotent stem cells for modeling Parkinson’s disease. World J. Stem Cell 11, 634–649 (2019).
Espay, A. J., Brundin, P. & Lang, A. E. Precision medicine for disease modification in Parkinson disease. Nat. Rev. Neurol. 13, 119–126 (2017).
Roos, R. A. C. et al. Huntington’s disease: a clinical review. Orphanet J. Rare Dis. 5, 40 (2010).
Geater, C., Hernandez, S., Thompson, L. & Mattis, V. B. in Huntington’s Disease. (eds Precious, S., Rosser, A. & Dunnett, S.) 41–73 (Humana, 2018). [Series Methods in Molecular Biology Vol. 1780].
Ledolter, J. & Kardon, R. H. Focus on data: statistical design of experiments and sample size selection using power analysis. Investig. Ophthalmol. Vis. Sci. 61, 11 (2020).
Regent, F. et al. Automation of human pluripotent stem cell differentiation toward retinal pigment epithelial cells for large-scale productions. Sci. Rep. 9, 10646 (2019).
Dhingra, A. et al. Automated production of human induced pluripotent stem cell-derived cortical and dopaminergic neurons with integrated live-cell monitoring. J. Vis. Exp. 2020, https://doi.org/10.3791/61525 (2020).
Virlogeux, A. et al. Reconstituting corticostriatal network on-a-chip reveals the contribution of the presynaptic compartment to Huntington’s disease. Cell Rep. 22, 110–122 (2018).
Zhuang, P., Sun, A. X., An, J., Chua, C. K. & Chew, S. Y. 3D neural tissue models: from spheroids to bioprinting. Biomaterials 154, 113–133 (2018).
Salaris, F. & Rosa, A. Construction of 3D in vitro models by bioprinting human pluripotent stem cells: challenges and opportunities. Brain Res. 1723, 146393 (2019).
Low, L. A., Mummery, C., Berridge, B. R., Austin, C. P. & Tagle, D. A. Organs-on-chips: into the next decade. Nat. Rev. Drug Discov. https://doi.org/10.1038/s41573-020-0079-3 (2020).
Kim, J., Koo, B. K. & Knoblich, J. A. Human organoids: model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 21, 571–584 (2020).
Miura, Y. et al. Generation of human striatal organoids and cortico-striatal assembloids from human pluripotent stem cells. Nat. Biotechnol. 38, 1421–1430 (2020).
Fortunato, A., Grainger, D. W. & Abou-El-Enein, M. Enhancing patient-level clinical data access to promote evidence-based practice and incentivize therapeutic innovation. Adv. Drug Deliv. Rev. 136–137, 97–104 (2018).
Hampel, H. et al. A precision medicine initiative for Alzheimer’s disease: the road ahead to biomarker-guided integrative disease modeling. Climacteric 20, 107–118 (2017).
Younesi, E. & Hofmann-Apitius, M. From integrative disease modeling to predictive, preventive, personalized and participatory (P4) medicine. EPMA J. 4, 23 (2013).
Ielapi, N. et al. Precision medicine and precision nursing: the era of biomarkers and precision health. Int. J. Gen. Med. 13, 1705 (2020).
Hampel, H. J., Bryant, S. E. O., Castrillo, J. I. & Ritchie, C. PRECISION MEDICINE - the golden gate for detection, treatment and prevention of Alzheimer’s disease. J. Prev. Alzheimers Dis. 3, 243–259 (2016).
Krzyszczyk, P. et al. The growing role of precision and personalized medicine for cancer treatment. Technology 6, 70–100 (2019).
Boufraqech, M. & Nilubol, N. Multi-omics signatures and translational potential to improve thyroid cancer patient outcome. Cancers 11, 1988 (2019).
Ardila, D. et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat. Med. 25, 954–961 (2019).
Rabbani, M. et al. Role of artificial intelligence in the care of patients with nonsmall cell lung cancer. Eur. J. Clin. Invest. 48, e12901 (2018).
Ramazzotti, D., Lal, A., Wang, B., Batzoglou, S. & Sidow, A. Multi-omic tumor data reveal diversity of molecular mechanisms that correlate with survival. Nat. Commun. 9, 4453 (2018).
Bonetto, V. et al. A systematic review of suggested molecular strata, biomarkers and their tissue sources in ALS. Front. Neurol. 10, 400 (2019).
Hampel, H. et al. Revolution of Alzheimer precision neurology. Passageway of systems biology and neurophysiology. J. Alzheimer’s Dis. 64, S47–S105 (2018).
Blinova, K. et al. Clinical trial in a dish: personalized stem cell-derived cardiomyocyte assay compared with clinical trial results for two QT-prolonging drugs. Clin. Transl. Sci. 12, 687–697 (2019).
Tucker, K. et al. Protecting patient privacy when sharing patient-level data from clinical trials. BMC Med. Res. Methodol. 16, 77 (2016).
Fröhlich, H. et al. From hype to reality: data science enabling personalized medicine. BMC Med. 16, 150 (2018).
Fujita, K. A. et al. Integrating pathways of Parkinson’s disease in a molecular interaction map. Mol. Neurobiol. 49, 88–102 (2014).
Mizuno, S. et al. AlzPathway: a comprehensive map of signaling pathways of Alzheimer’s disease. BMC Syst. Biol. 6, 52 (2012).
Scheffer, M. et al. Early-warning signals for critical transitions. Nature 461, 53–59 (2009).
Elger, C. E. & Lehnertz, K. Seizure prediction by non-linear time series analysis of brain electrical activity. Eur. J. Neurosci. 10, 786–789 (1998).
Mormann, F., Andrzejak, R. G., Elger, C. E. & Lehnertz, K. Seizure prediction: the long and winding road. Brain 130, 314–333 (2007).
Cook, M. J. et al. Prediction of seizure likelihood with a long-term, implanted seizure advisory system in patients with drug-resistant epilepsy: a first-in-man study. Lancet Neurol. 12, 563–571 (2013).
Kuhlmann, L., Lehnertz, K., Richardson, M. P., Schelter, B. & Zaveri, H. P. Seizure prediction – ready for a new era. Nat. Rev. Neurol. 14, 618–630 (2018).
Chen, L., Liu, R., Liu, Z. P., Li, M. & Aihara, K. Detecting early-warning signals for sudden deterioration of complex diseases by dynamical network biomarkers. Sci. Rep. 2, 342 (2012).
Liu, X. et al. Quantifying critical states of complex diseases using single-sample dynamic network biomarkers. PLoS Comput. Biol. 13, e1005633 (2017).
Kay, C., Hayden, M. R. & Leavitt, B. R. in Huntington Disease Ch. 3 (eds Feigin, S. A. & Anderson, K. E.) 31–46 (Elsevier, 2017). [Series Handbook of Clinical Neurology Vol. 144].
Landwehrmeyer, G. B. et al. Data analytics from Enroll-HD, a global clinical research platform for Huntington’s disease. Mov. Disord. Clin. Pract. 4, 212–224 (2017).
Ross, C. A. & Tabrizi, S. J. Huntington’s disease: from molecular pathogenesis to clinical treatment. Lancet Neurol. 10, 83–98 (2011).
Duyao, M. et al. Trinucleotide repeat length instability and age of onset in Huntington’s disease. Nat. Genet. 4, 387–392 (1993).
MacDonald, M. E. et al. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 72, 971–983 (1993).
Rosenblatt, A. et al. The association of CAG repeat length with clinical progression in Huntington disease. Neurology 66, 1016–1020 (2006).
Cannella, M. et al. The gender effect in juvenile Huntington disease patients of Italian origin. Am. J. Med. Genet. 125B, 92–98 (2004).
Swami, M. et al. Somatic expansion of the Huntington’s disease CAG repeat in the brain is associated with an earlier age of disease onset. Hum. Mol. Genet. 15, 3039–3047 (2009).
Kennedy, L. et al. Dramatic tissue-specific mutation length increases are an early molecular event in Huntington disease pathogenesis. Hum. Mol. Genet. 12, 3359–3367 (2003).
Langbehn, D. R. et al. A new model for prediction of the age of onset and penetrance for Huntington’s disease based on CAG length. Clin. Genet. 65, 267–277 (2004).
Wexler, N. S. et al. Venezuelan kindreds reveal that genetic and environmental factors modulate Huntington’s disease age of onset. Proc. Natl Acad. Sci. USA 101, 3498–3503 (2004).
Wright, G. E. B. et al. Interrupting sequence variants and age of onset in Huntington’s disease: clinical implications and emerging therapies. Lancet Neurol. 19, 930–939 (2020).
Wright, G. E. B. et al. Length of uninterrupted CAG, independent of polyglutamine size, results in increased somatic instability, hastening onset of Huntington disease. Am. J. Hum. Genet. 104, 1116–1126 (2019).
Lee, J. M. et al. CAG repeat not polyglutamine length determines timing of Huntington’s disease onset. Cell 178, 887–900.e14 (2019).
Li, J. L. et al. A genome scan for modifiers of age at onset in Huntington disease: the HD MAPS study. Am. J. Hum. Genet. 73, 682–687 (2003).
Lee, J. M. et al. Identification of genetic factors that modify clinical onset of Huntington’s disease. Cell 162, 516–526 (2015).
[No authors listed]. Unified Huntington’s disease rating scale: reliability and consistency. Mov. Disord. 11, 136–142 (1996).
Franciosi, S., Shim, Y., Lau, M., Hayden, M. R. & Leavitt, B. R. A systematic review and meta-analysis of clinical variables used in Huntington disease research. Mov. Disord. 28, 1987–1994 (2013).
Reilmann, R. & Schubert, R. in Huntington Disease Ch. 18 (eds Feigin, S. A. & Anderson, K. E.) 209–225 (Elsevier, 2017). [Series Handbook of Clinical Neurology Vol 144].
Caligiuri, M., Snell, C., Park, S. & Corey-Bloom, J. Handwriting movement abnormalities in symptomatic and premanifest Huntington’s disease. Mov. Disord. Clin. Pract. 6, 586–592 (2019).
Byrne, L. M. et al. Evaluation of mutant huntingtin and neurofilament proteins as potential markers in Huntington’s disease. Sci. Transl. Med. 10, eaat7108 (2018).
Zeun, P., Scahill, R. I., Tabrizi, S. J. & Wild, E. J. Fluid and imaging biomarkers for Huntington’s disease. Mol. Cell. Neurosci. 97, 67–80 (2019).
Pflanz, C. P. et al. One-year changes in brain microstructure differentiate preclinical Huntington’s disease stages. NeuroImage Clin. 25, 102099 (2020).
Nance, M. A. in Huntington Disease Ch. 1 (eds Feigin, S. A. & Anderson, K. E.) 3–14 (Elsevier, 2017). [Series Handbook of Clinical Neurology Vol. 144].
Wijeratne, P. A. et al. Robust markers and sample sizes for multicenter trials of Huntington disease. Ann. Neurol. 87, 751–762 (2020).
Jeon, I. et al. Neuronal properties, in vivo effects, and pathology of a Huntington’s disease patient-derived induced pluripotent stem cells. Stem Cell 30, 2054–2062 (2012).
An, M. C. et al. Genetic correction of Huntington’s disease phenotypes in induced pluripotent stem cells. Cell Stem Cell 11, 253–263 (2012).
Chae, J. I. L. et al. Quantitative proteomic analysis of induced pluripotent stem cells derived from a human Huntington’s disease patient. Biochem. J. 446, 359–371 (2012).
Sassone, J., Papadimitriou, E. & Thomaidou, D. Regenerative approaches in Huntington’s disease: from mechanistic insights to therapeutic protocols. Front. Neurosci. 12, 800 (2018).
Conforti, P. et al. Faulty neuronal determination and cell polarization are reverted by modulating HD early phenotypes. Proc. Natl Acad. Sci. USA 115, E762–E771 (2018).
Wiatr, K., Szlachcic, W. J., Trzeciak, M., Figlerowicz, M. & Figiel, M. Huntington disease as a neurodevelopmental disorder and early signs of the disease in stem cells. Mol. Neurobiol. 55, 3351–3371 (2018).
Lim, R. G. et al. Developmental alterations in Huntington’s disease neural cells and pharmacological rescue in cells and mice. Nat. Neurosci. 20, 648–660 (2017).
Haremaki, T. et al. Self-organizing neuruloids model developmental aspects of Huntington’s disease in the ectodermal compartment. Nat. Biotechnol. 37, 1198–1208 (2019).
Moss, D. J. H. et al. Identification of genetic variants associated with Huntington’s disease progression: a genome-wide association study. Lancet Neurol. 16, 701–711 (2017).
Böhnke, L., Traxler, L., Herdy, J. R. & Mertens, J. Human neurons to model aging: a dish best served old. Drug Discov. Today Dis. Models 27, 43–49 (2018).
Sidhaye, J. & Knoblich, J. A. Brain organoids: an ensemble of bioassays to investigate human neurodevelopment and disease. Cell Death Differ. 28, 52–67 (2021).
Rifes, P. et al. Modeling neural tube development by differentiation of human embryonic stem cells in a microfluidic WNT gradient. Nat. Biotechnol. 38, 1265–1273 (2020).
Vicente, J. et al. Mechanistic model-informed proarrhythmic risk assessment of drugs: review of the “CiPA” initiative and design of a prospective clinical validation study. Clin. Pharmacol. Ther. 103, 54–66 (2018).
Blinova, K. et al. Clinical trial in a dish: personalized stem cell derived cardiomyocyte assay compared to clinical trial results for two QT prolonging drugs. Clin. Transl. Sci. 12, 687–697 (2019).
Biggs, R., Carpenter, S. R. & Brock, W. A. Turning back from the brink: detecting an impending regime shift in time to avert it. Proc. Natl Acad. Sci. USA 106, 826–831 (2009).
Nakagawa, M. et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 26, 101–106 (2008).
Okita, K., Ichisaka, T. & Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 448, 313–317 (2007).
Rowland, B. D. & Peeper, D. S. KLF4, p21 and context-dependent opposing forces in cancer. Nat. Rev. Cancer 6, 11–23 (2006).
Merkle, F. T. et al. Human pluripotent stem cells recurrently acquire and expand dominant negative P53 mutations. Nature 545, 229–233 (2017).
Adhikary, S. & Eilers, M. Transcriptional regulation and transformation by Myc proteins. Nat. Rev. Mol. Cell Biol. 6, 635–645 (2005).
Zheng, W., Wang, Y., Chang, T., Huang, H. & Yee, J. K. Significant differences in genotoxicity induced by retrovirus integration in human T cells and induced pluripotent stem cells. Gene 519, 142–149 (2013).
D’Antonio, M. et al. Insights into the mutational burden of human induced pluripotent stem cells from an integrative multi-omics approach. Cell Rep. 24, 883–894 (2018).
Ma, H. et al. Abnormalities in human pluripotent cells due to reprogramming mechanisms. Nature 511, 177–183 (2014).
Strässler, E. T., Aalto-Setälä, K., Kiamehr, M., Landmesser, U. & Kränkel, N. Age is relative–impact of donor age on induced pluripotent stem cell-derived cell functionality. Front. Cardiovasc. Med. 5, 4 (2018).
Lister, R. et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature 471, 68–73 (2011).
Hiura, H. et al. Stability of genomic imprinting in human induced pluripotent stem cells. BMC Genet. 14, 32 (2013).
Pick, M. et al. Clone- and gene-specific aberrations of parental imprinting in human induced pluripotent stem cell. Stem Cells 27, 2686–2690 (2009).
Perrera, V. & Martello, G. How does reprogramming to pluripotency affect genomic imprinting? Front. Cell Dev. Biol. 7, 76 (2019).
Anguera, M. C. et al. Molecular signatures of human induced pluripotent stem cells highlight sex differences and cancer genes. Cell Stem Cell 11, 75–90 (2012).
Vallot, C. et al. Erosion of X chromosome inactivation in human pluripotent cells initiates with XACT coating and depends on a specific heterochromatin landscape. Cell Stem Cell 16, 533–546 (2015).
Ichida, J. K. & Kiskinis, E. Probing disorders of the nervous system using reprogramming approaches. EMBO J. 34, 1456–1477 (2015).
Nekrasov, E. D. et al. Manifestation of Huntington’s disease pathology in human induced pluripotent stem cell-derived neurons. Mol. Neurodegener. 11, 27 (2016).
Sullivan, S. et al. Quality control guidelines for clinical-grade human induced pluripotent stem cell lines. Regen. Med. 13, 859–866 (2018).
King, T. J. & Briggs, R. Changes in the nuclei of differentiating gastrula cells, as demonstrated by nuclear transplantation. Proc. Natl Acad. Sci. USA 41, 321–325 (1955).
Gurdon, J. B., Elsdale, T. R. & Fischberg, M. Sexually mature individuals of Xenopus laevis from the transplantation of single somatic nuclei. Nature 182, 64–65 (1958).
McGrath, J. & Solter, D. Nuclear transplantation in the mouse embryo by microsurgery and cell fusion. Science 220, 1300–1302 (1983).
Campbell, K. H. S., McWhir, J., Ritchie, W. A. & Wilmut, I. Sheep cloned by nuclear transfer from a cultured cell line. Nature 380, 64–66 (1996).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Davis, R. L., Weintraub, H. & Lassar, A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell 51, 987–1000 (1987).
Vierbuchen, T. et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463, 1035–1041 (2010).
Mandai, M. et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N. Engl. J. Med. 376, 1038–1046 (2017).
Mallapaty, S. Revealed: two men in China were first to receive pioneering stem-cell treatment for heart disease. Nature 581, 249–250 (2020).
Acknowledgements
The work of the authors was funded by the CHDI Foundation (JSC A11103), a non-profit biomedical research organization exclusively dedicated to developing therapeutics that will substantially improve the life of individuals affected by HD; Novel Strategies for Cell based Neural Reconstruction 2020-23 (NSC-reconstruct), the European Union’s Horizon 2020 Research and Innovation Programme grant agreement no. 874758; Fondazione Telethon – Italy (Grant no. GGP17102); and Programmi di Ricerca Scientifica di rilevanza Nazionale, grant 2008JKSHKN_001 and Prot. 2015AY9AYB Ministero dell’Università e della Ricerca Scientifica.
Author information
Authors and Affiliations
Contributions
P.R.d.V.C., D.B. and P.C. researched data for the article. P.R.d.V.C., D.B. and E.C. made a substantial contribution to discussion of content, wrote the article, and reviewed and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Neurology thanks V. Khurana, who co-reviewed with S. Srinivasan, P. De Sousa, C. Svendsen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
California Institute for Regenerative Medicine iPSC repository: https://www.cirm.ca.gov/researchers/ipsc-repository
ClinicalTrials.gov: www.clinicaltrials.gov
European Bank for induced Pluripotent Stem Cells: www.ebisc.org
Human Induced Pluripotent Stem Cell Initiative: www.hipsci.org
New York Stem Cell Foundation repository: https://nyscf.org/research-institute/repository-stem-cell-search/
RIKEN BRC Cell Bank: https://cell.brc.riken.jp/en/
WHO International Clinical Trials Registry Platform: https://www.who.int/clinical-trials-registry-platform
WiCell Research Institute: www.wicell.org/
Glossary
- Non-integrative delivery systems
-
Systems that favour the transient expression of reprogramming factors without their genomic integration.
- OncomiRs
-
Cancer-associated microRNAs.
Rights and permissions
About this article
Cite this article
Rivetti di Val Cervo, P., Besusso, D., Conforti, P. et al. hiPSCs for predictive modelling of neurodegenerative diseases: dreaming the possible. Nat Rev Neurol 17, 381–392 (2021). https://doi.org/10.1038/s41582-021-00465-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-021-00465-0
This article is cited by
-
ARSACS: Clinical Features, Pathophysiology and iPS-Derived Models
The Cerebellum (2025)
-
Stem cell therapies in stroke rehabilitation: a narrative review of current strategies and future prospects
The Egyptian Journal of Neurology, Psychiatry and Neurosurgery (2024)
-
Blood–brain barrier alterations and their impact on Parkinson’s disease pathogenesis and therapy
Translational Neurodegeneration (2024)
-
Biological basis of critical illness subclasses: from the bedside to the bench and back again
Critical Care (2024)
-
Neuropathogenesis-on-chips for neurodegenerative diseases
Nature Communications (2024)