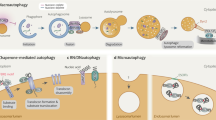
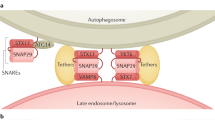
Abstract
Autophagy is a process that targets various intracellular elements for degradation. Autophagy can be non-selective — associated with the indiscriminate engulfment of cytosolic components — occurring in response to nutrient starvation and is commonly referred to as bulk autophagy. By contrast, selective autophagy degrades specific targets, such as damaged organelles (mitophagy, lysophagy, ER-phagy, ribophagy), aggregated proteins (aggrephagy) or invading bacteria (xenophagy), thereby being importantly involved in cellular quality control. Hence, not surprisingly, aberrant selective autophagy has been associated with various human pathologies, prominently including neurodegeneration and infection. In recent years, considerable progress has been made in understanding mechanisms governing selective cargo engulfment in mammals, including the identification of ubiquitin-dependent selective autophagy receptors such as p62, NBR1, OPTN and NDP52, which can bind cargo and ubiquitin simultaneously to initiate pathways leading to autophagy initiation and membrane recruitment. This progress opens the prospects for enhancing selective autophagy pathways to boost cellular quality control capabilities and alleviate pathology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
De Duve, C. & Wattiaux, R. Functions of lysosomes. Annu. Rev. Physiol. 28, 435–492 (1966).
Dikic, I. & Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 19, 349–364 (2018).
Jiang, P. & Mizushima, N. Autophagy and human diseases. Cell Res. 24, 69–79 (2014).
Ohsumi, Y. Molecular mechanism of autophagy in yeast, Saccharomyces cerevisiae. Phil. Trans. R. Soc. Lond. B 354, 1577–1580 (1999).
Hamasaki, M. et al. Autophagosomes form at ER-mitochondria contact sites. Nature 495, 389–393 (2013).
Lamb, C. A., Yoshimori, T. & Tooze, S. A. The autophagosome: origins unknown, biogenesis complex. Nat. Rev. Mol. Cell Biol. 14, 759–774 (2013).
Gubas, A. & Dikic, I. A guide to the regulation of selective autophagy receptors. FEBS J. 289, 75–89 (2022).
Johansen, T. & Lamark, T. Selective autophagy: ATG8 family proteins, LIR motifs and cargo receptors. J. Mol. Biol. 432, 80–103 (2020).
Kirkin, V. & Rogov, V. V. A diversity of selective autophagy receptors determines the specificity of the autophagy pathway. Mol. Cell 76, 268–285 (2019).
Nakagawa, I. et al. Autophagy defends cells against invading Group A Streptococcus. Science 306, 1037–1040 (2004).
Pan, J.-A. et al. TRIM21 ubiquitylates SQSTM1/p62 and suppresses protein sequestration to regulate redox homeostasis. Mol. Cell 61, 720–733 (2016).
Lee, Y. et al. Keap1/Cullin3 modulates p62/SQSTM1 activity via UBA domain ubiquitination. Cell Rep. 19, 188–202 (2017).
Pickles, S., Vigié, P. & Youle, R. J. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr. Biol. 28, R170–R185 (2018).
Tanaka, K. The PINK1-Parkin axis: an overview. Neurosci. Res. 159, 9–15 (2020).
Narendra, D., Tanaka, A., Suen, D. F. & Youle, R. J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 183, 795–803 (2008). The authors demonstrate the novel role of Parkin in mitophagy. Parkin is selectively recruited to damaged mitochondria and is critical for their autophagic degradation.
Jin, S. M. et al. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J. Cell Biol. 191, 933–942 (2010).
Sekine, S. & Youle, R. J. PINK1 import regulation; a fine system to convey mitochondrial stress to the cytosol. BMC Biol. 16, 2 (2018).
Yamano, K. & Youle, R. J. PINK1 is degraded through the N-end rule pathway. Autophagy 9, 1758–1769 (2013).
Deas, E. et al. PINK1 cleavage at position A103 by the mitochondrial protease PARL. Hum. Mol. Genet. 20, 867–879 (2011).
Kane, L. A. et al. PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. J. Cell Biol. 205, 143–153 (2014).
Koyano, F. et al. Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature 510, 162–166 (2014).
Shiba-Fukushima, K. et al. Phosphorylation of mitochondrial polyubiquitin by PINK1 promotes Parkin mitochondrial tethering. PLoS Genet. 10, e1004861 (2014).
Kazlauskaite, A. et al. Parkin is activated by PINK1-dependent phosphorylation of ubiquitin at Ser65. Biochem. J. 460, 127–139 (2014).
Bingol, B. et al. The mitochondrial deubiquitinase USP30 opposes parkin-mediated mitophagy. Nature 510, 370–375 (2014).
Kondapalli, C. et al. PINK1 is activated by mitochondrial membrane potential depolarization and stimulates Parkin E3 ligase activity by phosphorylating Serine 65. Open Biol. 2, 120080 (2012).
Narendra, D. P. et al. PINK1 is selectively stabilized on impaired mitochondria to activate parkin. PLoS Biol. 8, e1000298 (2010).
Gladkova, C., Maslen, S. L., Skehel, J. M. & Komander, D. Mechanism of parkin activation by PINK1. Nature 559, 410–414 (2018).
Sauvé, V. et al. Mechanism of parkin activation by phosphorylation. Nat. Struct. Mol. Biol. 25, 623–630 (2018).
Sarraf, S. A. et al. Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature 496, 372–376 (2013).
Ordureau, A. et al. Quantitative proteomics reveal a feedforward mechanism for mitochondrial PARKIN translocation and ubiquitin chain synthesis. Mol. Cell 56, 360–375 (2014).
Ordureau, A. et al. Dynamics of PARKIN-dependent mitochondrial ubiquitylation in induced neurons and model systems revealed by digital snapshot proteomics. Mol. Cell 70, 211–227.e8 (2018).
Tanaka, A. et al. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J. Cell Biol. 191, 1367–1380 (2010).
Yamano, K., Fogel, A. I., Wang, C., van der Bliek, A. M. & Youle, R. J. Mitochondrial Rab GAPs govern autophagosome biogenesis during mitophagy. eLife 3, e01612 (2014).
Yamano, K. et al. Endosomal Rab cycles regulate Parkin-mediated mitophagy. eLife 7, e31326 (2018).
Heo, J.-M. et al. RAB7A phosphorylation by TBK1 promotes mitophagy via the PINK-PARKIN pathway. Sci. Adv. 4, eaav0443 (2018).
Lazarou, M. et al. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524, 309–314 (2015).
Heo, J.-M., Ordureau, A., Paulo, J. A., Rinehart, J. & Harper, J. W. The PINK1-PARKIN mitochondrial ubiquitylation pathway drives a program of OPTN/NDP52 recruitment and TBK1 activation to promote mitophagy. Mol. Cell 60, 7–20 (2015).
Moore, A. S. & Holzbaur, E. L. F. Dynamic recruitment and activation of ALS-associated TBK1 with its target optineurin are required for efficient mitophagy. Proc. Natl Acad. Sci. USA 113, E3349–E3358 (2016).
Swatek, K. N. et al. Insights into ubiquitin chain architecture using Ub-clipping. Nature 572, 533–537 (2019).
Evans, C. S. & Holzbaur, E. L. Degradation of engulfed mitochondria is rate-limiting in Optineurin-mediated mitophagy in neurons. eLife 9, e50260 (2020).
Burman, J. L. et al. Mitochondrial fission facilitates the selective mitophagy of protein aggregates. J. Cell Biol. 216, 3231–3247 (2017).
Thurston, T. L. M., Ryzhakov, G., Bloor, S., von Muhlinen, N. & Randow, F. The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin-coated bacteria. Nat. Immunol. 10, 1215–1221 (2009).
Ravenhill, B. J. et al. The cargo receptor NDP52 initiates selective autophagy by recruiting the ULK complex to cytosol-invading bacteria. Mol. Cell 74, 320–329.e6 (2019). This study shows that FIP200, NDP52 and SINTBAD/NAP1 form a trimeric complex crucial for the initiation of xenophagy and that NDP52 directly recruits TBK1 and the ULK1 complex to invading bacteria.
Wild, P. et al. Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science 333, 228–233 (2011).
Richter, B. et al. Phosphorylation of OPTN by TBK1 enhances its binding to Ub chains and promotes selective autophagy of damaged mitochondria. Proc. Natl Acad. Sci. USA 113, 4039–4044 (2016).
Vargas, J. N. S. et al. Spatiotemporal control of ULK1 activation by NDP52 and TBK1 during selective autophagy. Mol. Cell 74, 347–362.e6 (2019). This study shows that NDP52 binds to FIP200, which allows NDP52 to localize the ULK1 complex selectively to damaged mitochondria downstream of Parkin activation to initiate mitophagy. Additionally, this study shows that TBK1 activity fosters the association of NDP52 with the ULK1 complex.
Terešak, P. et al. Regulation of PRKN-independent mitophagy. Autophagy 18, 24–39 (2022).
Zhang, J. & Ney, P. A. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ. 16, 939–946 (2009).
Sandoval, H. et al. Essential role for Nix in autophagic maturation of erythroid cells. Nature 454, 232–235 (2008).
Novak, I. et al. Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 11, 45–51 (2010).
Hanna, R. A. et al. Microtubule-associated protein 1 light chain 3 (LC3) interacts with Bnip3 protein to selectively remove endoplasmic reticulum and mitochondria via autophagy. J. Biol. Chem. 287, 19094–19104 (2012).
Princely Abudu, Y. et al. NIPSNAP1 and NIPSNAP2 act as ‘Eat Me’ signals for mitophagy. Dev. Cell 49, 509–525.e12 (2019).
Li, Y. et al. BNIP3L/NIX-mediated mitophagy: molecular mechanisms and implications for human disease. Cell Death Dis. 13, 14 (2021).
Nguyen, T. N. et al. Atg8 family LC3/GABARAP proteins are crucial for autophagosome-lysosome fusion but not autophagosome formation during PINK1/Parkin mitophagy and starvation. J. Cell Biol. 215, 857–874 (2016). Using CRISPR-gene editing to knockout genes encoding the LC3/GABARAP family proteins, this work shows that these proteins are dispensable for autophagosome formation but are instead essential for the acidification of autophagosomes through their role in autophagosome–lysosome fusion.
Itakura, E., Kishi-Itakura, C., Koyama-Honda, I. & Mizushima, N. Structures containing Atg9A and the ULK1 complex independently target depolarized mitochondria at initial stages of Parkin-mediated mitophagy. J. Cell Sci. 125, 1488–1499 (2012).
Fu, T. et al. Structural and biochemical advances on the recruitment of the autophagy-initiating ULK and TBK1 complexes by autophagy receptor NDP52. Sci. Adv. 7, eabi6582 (2021).
Shi, X. et al. ULK complex organization in autophagy by a C-shaped FIP200 N-terminal domain dimer. J. Cell Biol. 219, e201911047 (2020).
Yamano, K. et al. Critical role of mitochondrial ubiquitination and the OPTN-ATG9A axis in mitophagy. J. Cell Biol. 219, e201912144 (2020). This study reveals that the association between OPTN and ATG9A is important for mitophagy, suggesting that OPTN localizes ATG9A vesicles to provide membranes for mitophagy.
O’Loughlin, T. et al. OPTN recruitment to a Golgi-proximal compartment regulates immune signalling and cytokine secretion. J. Cell Sci. 133, jcs239822 (2020).
Zachari, M. et al. Selective autophagy of mitochondria on a ubiquitin-endoplasmic-reticulum platform. Dev. Cell 50, 627–643.e5 (2019).
Heo, J.-M. et al. Integrated proteogenetic analysis reveals the landscape of a mitochondrial-autophagosome synapse during PARK2-dependent mitophagy. Sci. Adv. 5, eaay4624 (2019).
Bansal, M. et al. Optineurin promotes autophagosome formation by recruiting the autophagy-related Atg12-5-16L1 complex to phagophores containing the Wipi2 protein. J. Biol. Chem. 293, 132–147 (2018).
Chang, C. et al. Reconstitution of cargo-induced LC3 lipidation in mammalian selective autophagy. Sci. Adv. 7, eabg4922 (2021).
Shi, X., Chang, C., Yokom, A. L., Jensen, L. E. & Hurley, J. H. The autophagy adaptor ndp52 and the fip200 coiled-coil allosterically activate ulk1 complex membrane recruitment. eLife 9, e59099 (2020). Using in vitro reconstitution assays, this work shows that NDP52 binds to and allosterically stimulates the membrane-binding of FIP200 by inducing a dynamic conformation of the membrane-binding region of FIP200.
Kim, J., Kundu, M., Viollet, B. & Guan, K.-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13, 132–141 (2011).
Turco, E. et al. FIP200 claw domain binding to p62 promotes autophagosome formation at ubiquitin condensates. Mol. Cell 74, 330–346.e11 (2019). This study reports that p62 directly associates with the Claw domain of FIP200 to localize the ULK1 complex to ubiquitylated protein aggregates. Additionally, this study shows that the binding of p62 to FIP200 is mutually exclusive from the binding of p62 and LC3.
Turco, E. et al. Reconstitution defines the roles of p62, NBR1 and TAX1BP1 in ubiquitin condensate formation and autophagy initiation. Nat. Commun. 12, 5212 (2021).
Nguyen, T. N. et al. Unconventional initiation of PINK1/Parkin mitophagy by optineurin. Preprint at bioRxiv https://doi.org/10.1101/2022.08.14.503930 (2022).
Padman, B. S. et al. LC3/GABARAPs drive ubiquitin-independent recruitment of Optineurin and NDP52 to amplify mitophagy. Nat. Commun. 10, 408 (2019).
Valente, E. M. et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science 304, 1158–1160 (2004).
Kitada, T. et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392, 605–608 (1998).
Clark, I. E. et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 441, 1162–1166 (2006).
Park, J. et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 441, 1157–1161 (2006).
Cai, X., Xu, H. & Chen, Z. J. Prion-like polymerization in immunity and inflammation. Cold Spring Harb. Perspect. Biol. 9, a023580 (2017).
Wang, C. & Youle, R. J. The role of mitochondria in apoptosis. Annu. Rev. Genet. 43, 95–118 (2009).
Johnson, B. N., Berger, A. K., Cortese, G. P. & Lavoie, M. J. The ubiquitin E3 ligase parkin regulates the proapoptotic function of Bax. Proc. Natl Acad. Sci. USA 109, 6283–6288 (2012).
Bernardini, J. P. et al. Parkin inhibits BAK and BAX apoptotic function by distinct mechanisms during mitophagy. EMBO J. 38, e99916 (2019).
Ham, S. J. et al. Decision between mitophagy and apoptosis by Parkin via VDAC1 ubiquitination. Proc. Natl Acad. Sci. USA 117, 4281–4291 (2020).
Akira, S., Uematsu, S. & Takeuchi, O. Pathogen recognition and innate immunity. Cell 124, 783–801 (2006).
Youle, R. J. Mitochondria-Striking a balance between host and endosymbiont. Science 365, eaaw9855 (2019).
West, A. P. et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 520, 553–557 (2015).
Wu, J. & Chen, Z. J. Innate immune sensing and signaling of cytosolic nucleic acids. Annu. Rev. Immunol. 32, 461–488 (2014).
Sliter, D. A. et al. Parkin and PINK1 mitigate STING-induced inflammation. Nature 561, 258–262 (2018).
Moehlman, A. T. & Youle, R. J. Mitochondrial quality control and restraining innate immunity. Annu. Rev. Cell Dev. Biol. 36, 265–289 (2020).
Pickrell, A. M. & Youle, R. J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 85, 257–273 (2015).
Borsche, M. et al. Mitochondrial damage-associated inflammation highlights biomarkers in PRKN/PINK1 parkinsonism. Brain 143, 3041–3051 (2020).
Surmeier, D. J. Determinants of dopaminergic neuron loss in Parkinson’s disease. FEBS J. 285, 3657–3668 (2018).
Evans, C. S. & Holzbaur, E. L. F. Autophagy and mitophagy in ALS. Neurobiol. Dis. 122, 35–40 (2019).
Van Laar, V. S. & Berman, S. B. The interplay of neuronal mitochondrial dynamics and bioenergetics: implications for Parkinson’s disease. Neurobiol. Dis. 51, 43–55 (2013).
Aschrafi, A. et al. A heterogeneous population of nuclear-encoded mitochondrial mRNAs is present in the axons of primary sympathetic neurons. Mitochondrion 30, 18–23 (2016).
Mandal, A. & Drerup, C. M. Axonal transport and mitochondrial function in neurons. Front. Cell Neurosci. 13, 373 (2019).
Coffey, J. W. & De Duve, C. Digestive activity of lysosomes. I. The digestion of proteins by extracts of rat liver lysosomes. J. Biol. Chem. 243, 3255–3263 (1968).
Boya, P. & Kroemer, G. Lysosomal membrane permeabilization in cell death. Oncogene 27, 6434–6451 (2008).
Maejima, I. et al. Autophagy sequesters damaged lysosomes to control lysosomal biogenesis and kidney injury. EMBO J. 32, 2336–2347 (2013). This paper shows that the clearance of damaged lysosomes occurs by autophagy and coined the term lysophagy.
Stahl-Meyer, J., Stahl-Meyer, K. & Jäättelä, M. Control of mitosis, inflammation, and cell motility by limited leakage of lysosomes. Curr. Opin. Cell Biol. 71, 29–37 (2021).
Hung, Y.-H., Chen, L. M.-W., Yang, J.-Y. & Yuan Yang, W. Spatiotemporally controlled induction of autophagy-mediated lysosome turnover. Nat. Commun. 4, 2111 (2013). This paper shows damaged lysosomes are ubiquitylated and that autophagic turnover is induced, termed lysophagy.
Aman, Y. et al. Autophagy in healthy aging and disease. Nat. Aging 1, 634–650 (2021).
Papadopoulos, C. et al. VCP/p97 cooperates with YOD1, UBXD1 and PLAA to drive clearance of ruptured lysosomes by autophagy. EMBO J. 36, 135–150 (2017).
Eapen, V. V., Swarup, S., Hoyer, M. J., Paulo, J. A. & Harper, J. W. Quantitative proteomics reveals the selectivity of ubiquitin-binding autophagy receptors in the turnover of damaged lysosomes by lysophagy. eLife 10, e72328 (2021).
Chauhan, S. et al. TRIMs and galectins globally cooperate and TRIM16 and Galectin-3 co-direct autophagy in endomembrane damage homeostasis. Dev. Cell 39, 13–27 (2016).
Yoshida, Y. et al. Ubiquitination of exposed glycoproteins by SCFFBXO27 directs damaged lysosomes for autophagy. Proc. Natl Acad. Sci. USA 114, 8574–8579 (2017).
Kravić, B. et al. Ubiquitin profiling of lysophagy identifies actin stabilizer CNN2 as a target of VCP/p97 and uncovers a link to HSPB1. Mol. Cell 82, 2633–2649.e7 (2022).
Koerver, L. et al. The ubiquitin-conjugating enzyme UBE2QL1 coordinates lysophagy in response to endolysosomal damage. EMBO Rep. 20, e48014 (2019).
Zhang, L., Sheng, R. & Qin, Z. The lysosome and neurodegenerative diseases. Acta Biochim. Biophys. Sin. 41, 437–445 (2009).
Papadopoulos, C. & Meyer, H. Detection and clearance of damaged lysosomes by the endo-lysosomal damage response and lysophagy. Curr. Biol. 27, R1330–R1341 (2017).
McBrayer, M. & Nixon, R. A. Lysosome and calcium dysregulation in Alzheimer’s disease: partners in crime. Biochem. Soc. Trans. 41, 1495–1502 (2013).
Lamark, T. & Johansen, T. Aggrephagy: selective disposal of protein aggregates by macroautophagy. Int. J. Cell Biol. 2012, 736905 (2012).
Menzies, F. M., Fleming, A. & Rubinsztein, D. C. Compromised autophagy and neurodegenerative diseases. Nat. Rev. Neurosci. 16, 345–357 (2015).
Scotter, E. L. et al. Differential roles of the ubiquitin proteasome system and autophagy in the clearance of soluble and aggregated TDP-43 species. J. Cell Sci. 127, 1263–1278 (2014).
Dikic, I. Proteasomal and autophagic degradation systems. Annu. Rev. Biochem. 86, 193–224 (2017).
Bjørkøy, G. et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J. Cell Biol. 171, 603–614 (2005). This paper initially identifie the LIR domain and highlighted, for the first time, the role of p62 in aggrephagy.
Pankiv, S. et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 282, 24131–24145 (2007).
Wurzer, B. et al. Oligomerization of p62 allows for selection of ubiquitinated cargo and isolation membrane during selective autophagy. eLife 4, e08941 (2015).
Zaffagnini, G. et al. p62 filaments capture and present ubiquitinated cargos for autophagy. EMBO J. 37, e98308 (2018).
Clausen, T. H. et al. p62/SQSTM1 and ALFY interact to facilitate the formation of p62 bodies/ALIS and their degradation by autophagy. Autophagy 6, 330–344 (2010).
Liu, X. et al. The BEACH-containing protein WDR81 coordinates p62 and LC3C to promote aggrephagy. J. Cell Biol. 216, 1301–1320 (2017).
Matsumoto, G., Shimogori, T., Hattori, N. & Nukina, N. TBK1 controls autophagosomal engulfment of polyubiquitinated mitochondria through p62/SQSTM1 phosphorylation. Hum. Mol. Genet. 24, 4429–4442 (2015).
Savova, A., Romanov, J. & Martens, S. NBR1 directly promotes the formation of p62–ubiquitin condensates via its PB1 and UBA domains. Preprint at bioRxiv https://doi.org/10.1101/2020.09.18.303552 (2020).
Sun, D., Wu, R., Li, P. & Yu, L. Phase separation in regulation of aggrephagy. J. Mol. Biol. 432, 160–169 (2020).
Sun, D., Wu, R., Zheng, J., Li, P. & Yu, L. Polyubiquitin chain-induced p62 phase separation drives autophagic cargo segregation. Cell Res. 28, 405–415 (2018).
Kirkin, V. et al. A role for NBR1 in autophagosomal degradation of ubiquitinated substrates. Mol. Cell 33, 505–516 (2009).
Long, J. et al. Dimerisation of the UBA domain of p62 inhibits ubiquitin binding and regulates NF-κB signalling. J. Mol. Biol. 396, 178–194 (2010).
Walinda, E. et al. Solution structure of the ubiquitin-associated (UBA) domain of human autophagy receptor NBR1 and its interaction with ubiquitin and polyubiquitin. J. Biol. Chem. 289, 13890–13902 (2014).
Agudo-Canalejo, J. et al. Wetting regulates autophagy of phase-separated compartments and the cytosol. Nature 591, 142–146 (2021).
Schultz, S. W. et al. Should I bend or should I grow: the mechanisms of droplet-mediated autophagosome formation. Autophagy 17, 1046–1048 (2021).
Sarraf, S. A. et al. Loss of TAX1BP1-directed autophagy results in protein aggregate accumulation in the brain. Mol. Cell 80, 779–795.e10 (2020).
Ohnstad, A. E. et al. Receptor-mediated clustering of FIP200 bypasses the role of LC3 lipidation in autophagy. EMBO J. 39, e104948 (2020).
Lu, K., Psakhye, I. & Jentsch, S. Autophagic clearance of PolyQ proteins mediated by ubiquitin-Atg8 adaptors of the conserved CUET protein family. Cell 158, 549–563 (2014).
Ryan, T. A. et al. Tollip coordinates Parkin-dependent trafficking of mitochondrial-derived vesicles. EMBO J. 39, e102539 (2020).
Zellner, S., Schifferer, M. & Behrends, C. Systematically defining selective autophagy receptor-specific cargo using autophagosome content profiling. Mol. Cell 81, 1337–1354.e8 (2021).
Jo, C. et al. Nrf2 reduces levels of phosphorylated tau protein by inducing autophagy adaptor protein NDP52. Nat. Commun. 5, 3496 (2014).
Pickford, F. et al. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J. Clin. Invest. 118, 2190–2199 (2008).
Ravikumar, B. et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat. Genet. 36, 585–595 (2004).
Winslow, A. R. et al. α-Synuclein impairs macroautophagy: implications for Parkinson’s disease. J. Cell Biol. 190, 1023–1037 (2010).
Buchan, J. R., Kolaitis, R.-M., Taylor, J. P. & Parker, R. Eukaryotic stress granules are cleared by autophagy and Cdc48/VCP function. Cell 153, 1461–1474 (2013).
Shibutani, S. T., Saitoh, T., Nowag, H., Münz, C. & Yoshimori, T. Autophagy and autophagy-related proteins in the immune system. Nat. Immunol. 16, 1014–1024 (2015).
Dong, X. & Levine, B. Autophagy and viruses: adversaries or allies? J. Innate Immun. 5, 480–493 (2013).
Choy, A. et al. The Legionella effector RavZ inhibits host autophagy through irreversible Atg8 deconjugation. Science 338, 1072–1076 (2012).
Ogawa, M. et al. Escape of intracellular Shigella from autophagy. Science 307, 727–731 (2005).
Xu, Y. et al. A bacterial effector reveals the V-ATPase-ATG16L1 axis that initiates xenophagy. Cell 178, 552–566.e20 (2019).
Xu, Y. et al. ARF GTPases activate Salmonella effector SopF to ADP-ribosylate host V-ATPase and inhibit endomembrane damage-induced autophagy. Nat. Struct. Mol. Biol. 29, 67–77 (2022).
Cemma, M. & Brumell, J. H. Interactions of pathogenic bacteria with autophagy systems. Curr. Biol. 22, R540–R545 (2012).
Birmingham, C. L., Smith, A. C., Bakowski, M. A., Yoshimori, T. & Brumell, J. H. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J. Biol. Chem. 281, 11374–11383 (2006).
Biering, S. B. et al. Viral replication complexes are targeted by LC3-guided interferon-inducible GTPases. Cell Host Microbe 22, 74–85.e7 (2017).
Sagnier, S. et al. Autophagy restricts HIV-1 infection by selectively degrading Tat in CD4 + T lymphocytes. J. Virol. 89, 615–625 (2015).
Birmingham, C. L. & Brumell, J. H. Autophagy recognizes intracellular Salmonella enterica serovar Typhimurium in damaged vacuoles. Autophagy 2, 156–158 (2006).
Perrin, A. J., Jiang, X., Birmingham, C. L., So, N. S. Y. & Brumell, J. H. Recognition of bacteria in the cytosol of Mammalian cells by the ubiquitin system. Curr. Biol. 14, 806–811 (2004).
Fujita, N. et al. Recruitment of the autophagic machinery to endosomes during infection is mediated by ubiquitin. J. Cell Biol. 203, 115–128 (2013). This study shows that a damaged endosomal membrane causes ubiquitin-coating and autophagosomal membrane formation on the targets for selective autophagy using the artificial polystyrene beads coated with transfection reagent, which damages the membrane.
Thurston, T. L. M., Wandel, M. P., von Muhlinen, N., Foeglein, Á. & Randow, F. Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature 482, 414–418 (2012). This study show that galectin 8 works as a danger receptor for invading bacteria. It promotes early recruitment of NDP52 to the invading bacteria via the galectin 8-binding domain of NDP52, which further facilitates subsequent binding of NDP52 with ubiquitin-coated bacteria.
Lin, C.-Y. et al. Autophagy receptor tollip facilitates bacterial autophagy by recruiting Galectin-7 in response to group A streptococcus infection. Front. Cell Infect. Microbiol. 10, 583137 (2020).
Chai, Q. et al. A Mycobacterium tuberculosis surface protein recruits ubiquitin to trigger host xenophagy. Nat. Commun. 10, 1973 (2019).
Yamada, A., Hikichi, M., Nozawa, T. & Nakagawa, I. FBXO2/SCF ubiquitin ligase complex directs xenophagy through recognizing bacterial surface glycan. EMBO Rep. 22, e52584 (2021).
Otten, E. G. et al. Ubiquitylation of lipopolysaccharide by RNF213 during bacterial infection. Nature 594, 111–116 (2021). This study shows that bacterial lipopolysaccharide, a non-proteinaceous substrate, is ubiquitylated upon bacterial invasion to promote their clearance by xenophagy.
von Muhlinen, N. et al. LC3C, bound selectively by a noncanonical LIR motif in NDP52, is required for antibacterial autophagy. Mol. Cell 48, 329–342 (2012).
Zheng, Y. T. et al. The adaptor protein p62/SQSTM1 targets invading bacteria to the autophagy pathway. J. Immunol. 183, 5909–5916 (2009). This is a classic study that establishes that receptor protein p62 is recruited to invading Salmonella and facilitate their autophagic degradation.
Verlhac, P. et al. Autophagy receptor NDP52 regulates pathogen-containing autophagosome maturation. Cell Host Microbe 17, 515–525 (2015).
Cemma, M., Kim, P. K. & Brumell, J. H. The ubiquitin-binding adaptor proteins p62/SQSTM1 and NDP52 are recruited independently to bacteria-associated microdomains to target Salmonella to the autophagy pathway. Autophagy 7, 341–345 (2011).
Tumbarello, D. A. et al. The autophagy receptor TAX1BP1 and the molecular motor myosin VI are required for clearance of Salmonella typhimurium by autophagy. PLoS Pathog. 11, e1005174 (2015).
Lin, C.-Y. et al. LAMTOR2/LAMTOR1 complex is required for TAX1BP1-mediated xenophagy. Cell Microbiol. 21, e12981 (2019).
Manzanillo, P. S. et al. The ubiquitin ligase parkin mediates resistance to intracellular pathogens. Nature 501, 512–516 (2013).
Franco, L. H. et al. The ubiquitin ligase Smurf1 functions in selective autophagy of Mycobacterium tuberculosis and anti-tuberculous host defense. Cell Host Microbe 21, 59–72 (2017).
Huett, A. et al. The LRR and RING domain protein LRSAM1 is an E3 ligase crucial for ubiquitin-dependent autophagy of intracellular Salmonella Typhimurium. Cell Host Microbe 12, 778–790 (2012).
Heath, R. J. et al. RNF166 determines recruitment of adaptor proteins during antibacterial autophagy. Cell Rep. 17, 2183–2194 (2016).
Fiskin, E., Bionda, T., Dikic, I. & Behrends, C. Global analysis of host and bacterial ubiquitinome in response to Salmonella typhimurium infection. Mol. Cell 62, 967–981 (2016).
Noad, J. et al. LUBAC-synthesized linear ubiquitin chains restrict cytosol-invading bacteria by activating autophagy and NF-κB. Nat. Microbiol. 2, 17063 (2017). This study shows that ubiquitin-coated invading Salmonella are subsequently labelled by linear, M1-linked polyubiquitin chains by LUBAC, promoting activation of the NF-κB signalling pathway.
Fuseya, Y. et al. The HOIL-1L ligase modulates immune signalling and cell death via monoubiquitination of LUBAC. Nat. Cell Biol. 22, 663–673 (2020).
Shahnazari, S. et al. A diacylglycerol-dependent signaling pathway contributes to regulation of antibacterial autophagy. Cell Host Microbe 8, 137–146 (2010).
Khaminets, A. et al. Regulation of endoplasmic reticulum turnover by selective autophagy. Nature 522, 354–358 (2015).
Grumati, P. et al. Full length RTN3 regulates turnover of tubular endoplasmic reticulum via selective autophagy. eLife 6, e25555 (2017).
Fumagalli, F. et al. Translocon component Sec62 acts in endoplasmic reticulum turnover during stress recovery. Nat. Cell Biol. 18, 1173–1184 (2016).
An, H. et al. TEX264 is an endoplasmic reticulum-resident ATG8-interacting protein critical for ER remodeling during nutrient stress. Mol. Cell 74, 891–908.e10 (2019).
Chino, H., Hatta, T., Natsume, T. & Mizushima, N. Intrinsically disordered protein TEX264 mediates ER-phagy. Mol. Cell 74, 909–921.e6 (2019).
Chen, Q. et al. ATL3 is a tubular ER-phagy receptor for GABARAP-mediated selective autophagy. Curr. Biol. 29, 846–855.e6 (2019).
Smith, M. D. et al. CCPG1 is a non-canonical autophagy cargo receptor essential for ER-phagy and pancreatic ER proteostasis. Dev. Cell 44, 217–232.e11 (2018).
Nthiga, T. M. et al. CALCOCO1 acts with VAMP-associated proteins to mediate ER-phagy. EMBO J. 39, e103649 (2020).
Yang, H. et al. Sequestosome 1/p62 protein is associated with autophagic removal of excess hepatic endoplasmic reticulum in mice. J. Biol. Chem. 291, 18663–18674 (2016).
Ji, C. H. et al. The N-degron pathway mediates ER-phagy. Mol. Cell 75, 1058–1072.e9 (2019).
Liang, J. R. et al. A genome-wide ER-phagy screen highlights key roles of mitochondrial metabolism and ER-resident UFMylation. Cell 180, 1160–1177.e20 (2020).
Stephani, M. et al. A cross-kingdom conserved ER-phagy receptor maintains endoplasmic reticulum homeostasis during stress. eLife 9, e58396 (2020).
Deosaran, E. et al. NBR1 acts as an autophagy receptor for peroxisomes. J. Cell Sci. 126, 939–952 (2013).
Yamashita, S., Abe, K., Tatemichi, Y. & Fujiki, Y. The membrane peroxin PEX3 induces peroxisome-ubiquitination-linked pexophagy. Autophagy 10, 1549–1564 (2014).
Kim, P. K., Hailey, D. W., Mullen, R. T. & Lippincott-Schwartz, J. Ubiquitin signals autophagic degradation of cytosolic proteins and peroxisomes. Proc. Natl Acad. Sci. USA 105, 20567–20574 (2008).
Sargent, G. et al. PEX2 is the E3 ubiquitin ligase required for pexophagy during starvation. J. Cell Biol. 214, 677–690 (2016).
Zhang, J. et al. ATM functions at the peroxisome to induce pexophagy in response to ROS. Nat. Cell Biol. 17, 1259–1269 (2015).
Zheng, J., Chen, X., Liu, Q., Zhong, G. & Zhuang, M. Ubiquitin ligase MARCH5 localizes to peroxisomes to regulate pexophagy. J. Cell Biol. 221, e202103156 (2022).
Hara-Kuge, S. & Fujiki, Y. The peroxin Pex14p is involved in LC3-dependent degradation of mammalian peroxisomes. Exp. Cell Res. 314, 3531–3541 (2008).
Nakatogawa, H. Spoon-feeding ribosomes to autophagy. Mol. Cell 71, 197–199 (2018).
An, H. & Harper, J. W. Systematic analysis of ribophagy in human cells reveals bystander flux during selective autophagy. Nat. Cell Biol. 20, 135–143 (2018).
Wyant, G. A. et al. NUFIP1 is a ribosome receptor for starvation-induced ribophagy. Science 360, 751–758 (2018).
An, H., Ordureau, A., Körner, M., Paulo, J. A. & Harper, J. W. Systematic quantitative analysis of ribosome inventory during nutrient stress. Nature 583, 303–309 (2020).
Maio, N. & Rouault, T. A. Outlining the complex pathway of mammalian Fe-S cluster biogenesis. Trends Biochem. Sci. 45, 411–426 (2020).
Kaur, J. & Debnath, J. Autophagy at the crossroads of catabolism and anabolism. Nat. Rev. Mol. Cell Biol. 16, 461–472 (2015).
Mancias, J. D., Wang, X., Gygi, S. P., Harper, J. W. & Kimmelman, A. C. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 509, 105–109 (2014). This work is the first to identify NCOA4 as a receptor protein for the autophagic degradation of ferritin.
Mancias, J. D. et al. Ferritinophagy via NCOA4 is required for erythropoiesis and is regulated by iron dependent HERC2-mediated proteolysis. eLife 4, e10308 (2015).
Goodwin, J. M. et al. Autophagy-independent lysosomal targeting regulated by ULK1/2-FIP200 and ATG9. Cell Rep. 20, 2341–2356 (2017).
Koutsifeli, P. et al. Glycogen-autophagy: molecular machinery and cellular mechanisms of glycophagy. J. Biol. Chem. 298, 102093 (2022).
Heden, T. D., Chow, L. S., Hughey, C. C. & Mashek, D. G. Regulation and role of glycophagy in skeletal muscle energy metabolism. Autophagy 18, 1078–1089 (2021).
Jiang, S. et al. Starch binding domain-containing protein 1/genethonin 1 is a novel participant in glycogen metabolism. J. Biol. Chem. 285, 34960–34971 (2010).
Jiang, S., Wells, C. D. & Roach, P. J. Starch-binding domain-containing protein 1 (Stbd1) and glycogen metabolism: identification of the Atg8 family interacting motif (AIM) in Stbd1 required for interaction with GABARAPL1. Biochem. Biophys. Res. Commun. 413, 420–425 (2011).
Han, Z. et al. Model-based analysis uncovers mutations altering autophagy selectivity in human cancer. Nat. Commun. 12, 3258 (2021).
Mellor, K. M., Varma, U., Stapleton, D. I. & Delbridge, L. M. D. Cardiomyocyte glycophagy is regulated by insulin and exposure to high extracellular glucose. Am. J. Physiol. Heart Circ. Physiol. 306, H1240–H1245 (2014).
Mellor, K. M. et al. Protective role Atg8 homologue Gabarapl1 regulating cardiomyocyte glycophagy in diabetic heart disease. Preprint at bioRxiv https://doi.org/10.1101/2021.06.21.449174 (2021).
Herker, E., Vieyres, G., Beller, M., Krahmer, N. & Bohnert, M. Lipid droplet contact sites in health and disease. Trends Cell Biol. 31, 345–358 (2021).
Olzmann, J. A. & Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 20, 137–155 (2019).
Tauchi-Sato, K., Ozeki, S., Houjou, T., Taguchi, R. & Fujimoto, T. The surface of lipid droplets is a phospholipid monolayer with a unique fatty acid composition. J. Biol. Chem. 277, 44507–44512 (2002).
Choudhary, V., Ojha, N., Golden, A. & Prinz, W. A. A conserved family of proteins facilitates nascent lipid droplet budding from the ER. J. Cell Biol. 211, 261–271 (2015).
Szymanski, K. M. et al. The lipodystrophy protein seipin is found at endoplasmic reticulum lipid droplet junctions and is important for droplet morphology. Proc. Natl Acad. Sci. USA 104, 20890–20895 (2007).
Singh, R. et al. Autophagy regulates lipid metabolism. Nature 458, 1131–1135 (2009).
Zechner, R., Madeo, F. & Kratky, D. Cytosolic lipolysis and lipophagy: two sides of the same coin. Nat. Rev. Mol. Cell Biol. 18, 671–684 (2017).
Schott, M. B. et al. Lipid droplet size directs lipolysis and lipophagy catabolism in hepatocytes. J. Cell Biol. 218, 3320–3335 (2019).
Robichaud, S. et al. Identification of novel lipid droplet factors that regulate lipophagy and cholesterol efflux in macrophage foam cells. Autophagy 17, 3671–3689 (2021).
Bersuker, K. et al. A proximity labeling strategy provides insights into the composition and dynamics of lipid droplet proteomes. Dev. Cell 44, 97–112.e7 (2018).
Wang, L. et al. Ethanol-triggered lipophagy requires SQSTM1 in AML12 hepatic cells. Sci. Rep. 7, 12307 (2017).
Berardi, D. et al. BNIP3 attenuates hepatocellular carcinoma by promoting lipid droplet turnover at the lysosome. Res. Sq. https://doi.org/10.21203/rs.3.rs-947988/v1 (2021).
Chang, C. et al. Reconstitution of cargo-induced LC3 lipidation in mammalian selective autophagy. Sci. Adv. 7, eabg4922 (2021).
Sawa-Makarska, J. et al. Reconstitution of autophagosome nucleation defines Atg9 vesicles as seeds for membrane formation. Science 369, eaaz7714 (2020).
Farré, J.-C. & Subramani, S. Mechanistic insights into selective autophagy pathways: lessons from yeast. Nat. Rev. Mol. Cell Biol. 17, 537–552 (2016).
Torggler, R. et al. Two independent pathways within selective autophagy converge to activate Atg1 kinase at the vacuole. Mol. Cell 64, 221–235 (2016).
Kamber, R. A., Shoemaker, C. J. & Denic, V. Receptor-bound targets of selective autophagy use a Scaffold protein to activate the Atg1 kinase. Mol. Cell 59, 372–381 (2015).
Pan, Z. Q. et al. Atg1 kinase in fission yeast is activated by atg11-mediated dimerization and cis-autophosphorylation. eLife 9, e58073 (2020).
Nakatogawa, H. Mechanisms governing autophagosome biogenesis. Nat. Rev. Mol. Cell Biol. 21, 439–458 (2020).
Takahashi, D. et al. AUTACs: cargo-specific degraders using selective autophagy. Mol. Cell 76, 797–810.e10 (2019).
Li, Z. et al. Allele-selective lowering of mutant HTT protein by HTT-LC3 linker compounds. Nature 575, 203–209 (2019).
Wong, Y. C. & Holzbaur, E. L. F. Optineurin is an autophagy receptor for damaged mitochondria in parkin-mediated mitophagy that is disrupted by an ALS-linked mutation. Proc. Natl Acad. Sci. USA 111, E4439–E4448 (2014).
Radulovic, M. et al. ESCRT-mediated lysosome repair precedes lysophagy and promotes cell survival. EMBO J. 37, e99753 (2018).
Medina, D. L. et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat. Cell Biol. 17, 288–299 (2015).
Nakamura, S. et al. LC3 lipidation is essential for TFEB activation during the lysosomal damage response to kidney injury. Nat. Cell Biol. 22, 1252–1263 (2020).
Papadopoulos, C., Kravic, B. & Meyer, H. Repair or lysophagy: dealing with damaged lysosomes. J. Mol. Biol. 432, 231–239 (2020).
Kluge, A. F. et al. Novel highly selective inhibitors of ubiquitin specific protease 30 (USP30) accelerate mitophagy. Bioorg. Med. Chem. Lett. 28, 2655–2659 (2018).
Ciechanover, A. & Kwon, Y. T. Degradation of misfolded proteins in neurodegenerative diseases: therapeutic targets and strategies. Exp. Mol. Med. 47, e147 (2015).
Meijer, A. J. & Codogno, P. Regulation and role of autophagy in mammalian cells. Int. J. Biochem. Cell Biol. 36, 2445–2462 (2004).
Burslem, G. M. & Crews, C. M. Proteolysis-targeting chimeras as therapeutics and tools for biological discovery. Cell 181, 102–114 (2020).
Acknowledgements
This work was supported by the Intramural Program of the National Institute of Neurological Disorders and Stroke.
Author information
Authors and Affiliations
Contributions
M.H., T.K. and T.Y. wrote and edited the sections on introduction, lysophagy and xenophagy, and created the figures and a table associated with these sections. J.N.S.V. and R.J.Y. wrote and edited the sections on mitophagy, aggrephagy, autophagy of other cellular structures, therapeutic opportunities, and conclusions and perspectives, and created the figures associated with these sections.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Molecular Cell Biology thanks Zvulun Elazar, Hong Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- LC3/GABARAP proteins
-
Mammalian paralogs of Atg8 protein. There are over six paralogs of Atg8 in mammals. The paralogs are activated by the processing of the C-terminal residue of LC3 paralogs, exposing G residue that is modified with phosphatidylethanolamine. LC3-phosphatidylethanolamine stably binds to autophagosome and facilitates autophagosome formation and maturation.
- TOM and TIM complex
-
A mitochondrial protein complexe that facilitates the translocation of cytosolic proteins containing a mitochondrial-targeting sequence into the mitochondria.
- p97
-
A protein, member of the AAA-ATPase, also known as valosin-containing protein (VCP) or cdc48.
- Reticulocyte
-
The immature form of red blood cells (erythrocytes) still containing RNA. It is generated from progenitor cells via erythropoiesis, a process accompanied by enucleation in mammals.
- ATG9A
-
A transmembrane protein with a phospholipid scramblase activity that plays a key role in the initiation of autophagy through the delivery of membranes to growing autophagosomes.
- ULK1 complex
-
A protein kinase complex consists of ULK1 kinase, ATG13, ATG101 and FIP200. It promotes the early step of autophagy by facilitating the formation of an isolation membrane.
- Mitochondrial antiviral signalling protein
-
(MAVS). A protein localized on the outer membrane of the mitochondria and activated by viral RNA, leading to increased levels of pro-inflammatory cytokines.
- VDAC1
-
A protein forming a voltage-dependent ion channel on the outer mitochondrial membrane. VDACs are responsible for the transport of nucleotides and metabolites from the cytosol into the mitochondria.
- Damage-associated molecular patterns
-
(DAMPs). Various molecules released during cell death via infection or damage. For instance, mitochondrial DNA released by apoptotic cells act as a DAMP and are recognized by Toll-like receptor 9 expressed by other cells, leading to inflammatory responses.
- Amyloid-β
-
A large transmembrane protein that accumulates in the brain of patients with Alzheimer disease forming amyloid plaques — suggested causative agents of neurodegeneration in Alzheimer disease.
- LLOMe
-
A dipeptide that is activated by lysosome enzyme like cathepsin and ruptures lysosomal membrane.
- Galectins
-
Proteins termed S-type lectins, which bind β-galactoside carbohydrates. They bind to glycoproteins on the inner membrane of endosomes; therefore, endosomal membrane rupture causes the accumulation of galectins, acting as a danger signal provoking selective autophagy.
- E3 ubiquitin ligases
-
Enzymes that selectively modify proteins by covalently attaching ubiquitin.
- Beclin 1
-
The mammalian homologue of yeast Atg6, a component of class III phosphoinositide 3-kinase (PI3K) complex. The BECN1 gene, encoding beclin 1, is located at a locus closed to the BRCA1 tumour suppressor gene and is therefore often deleted together with BRCA1 in breast cancer.
- ESCRT-III complex
-
Long filamentous complex promoting membrane remodelling, nucleation and scission to facilitate endocytosis or generation of multivesicular bodies together with other ESCRT complexes.
- Endo-lysosomal damage response
-
(ELDR). Cellular response triggered by lysosomal damage. ELDR complex contains ubiquitin-directed AAA-ATPase p97, deubiquitinating enzyme YOD1, and cofactors UBXD1 and PLAA.
- Multisystem proteinopathy
-
Defined as a combination of multiple degenerative disorders, such as amyotrophic lateral sclerosis (ALS) and inclusion body myopathy, characterized by the presence of protein aggregates in various organs, including muscle, bone and the central nervous system.
- E2 ubiquitin-conjugating enzyme
-
Enzyme that transfers ubiquitin from ubiquitin-activation enzyme E1 to a target protein through the support of E3.
- α-Synuclein
-
Neuronal protein that regulates synaptic vesicle trafficking and neurotransmitter release. Aggregates of α-synuclein establish insoluble fibrils, which are found in patients with Parkinson disease.
- Tau
-
Protein that functions to stabilize microtubules in axons. When hyperphosphorylated, it forms insoluble aggregates, causative of neurodegenerative diseases such as Alzheimer disease and Parkinson disease.
- Huntingtin
-
Protein involves in axonal transport. Mutants that show expansion of poly-glutamine repeats are causative of Huntington disease.
- Cytochrome c
-
Haeme protein attached to the inner membrane of a mitochondrion. In response to an apoptotic signal, it is released into the cytoplasm and activates caspase 9.
- Chaperone-mediated autophagy
-
A mode of autophagy that utilizes cytosolic HSC70 protein, which binds to targets and the lysosomal membrane protein LAMP2A. It is distinguished from macroautophagy, which is often referred to as just autophagy. Chaperone-mediated autophagy facilitates protein degradation via direct incorporation of proteins without the formation of the autophagosome.
- TDP43
-
An RNA-binding protein that is mutated in ALS. Furthermore, the aggregation of this protein is the neuropathological hallmark of ALS and frontotemporal dementia.
- FUS
-
A protein that functions as an RNA-binding protein. Mutations in FUS lead to early onset ALS.
- AMPK
-
A kinase activated upon stresses that reduce cellular ATP levels to promote a line of pathways that protect cells from the stresses. AMPK and mTOR cooperatively regulate autophagy.
- Cytosolic-to-vacuole targeting pathway
-
A pathway responsible for delivering some cytosolic proteins into vacuoles in yeast. Sharing certain components required for macroautophagy, cytosolic-to-vacuole targeting is considered one form of selective autophagy.
- Proteolysis-targeting chimaeras
-
Heterobifunctional molecules that target E3 ligase complexes to specific substrates to induce the ubiquitylation and subsequent proteasomal degradation of the target.
- Lipopolysaccharide
-
A major component of outer membranes of Gram-negative bacteria. It consists of lipid A, oligosaccharide and the O-antigen. The structure of lipid A and oligosaccharide is shared among many bacteria but that of O-antigen is variable.
- NEMO
-
A protein that is involved in the kinase complex, which facilitates activation of NF-κB. It phosphorylates IκB, invoking nuclear translocation of NF-κB.
- mTOR
-
A protein kinase that senses cellular metabolism, such as nutrients, energy and hormone levels, or exogenous stresses, thereby regulating a variety of cellular actions such as protein synthesis, proliferation, endocytosis and autophagy. mTOR binds to other subunits to form complexes called mTORC1 and mTORC2.
- Foam cells
-
Macrophages containing cholesterol and observed during arteriosclerotic vascular disease.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vargas, J.N.S., Hamasaki, M., Kawabata, T. et al. The mechanisms and roles of selective autophagy in mammals. Nat Rev Mol Cell Biol 24, 167–185 (2023). https://doi.org/10.1038/s41580-022-00542-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41580-022-00542-2
This article is cited by
-
Fasting activates optineurin-mediated mitophagy in chondrocytes to protect against osteoarthritis
Communications Biology (2025)
-
Comprehensive analysis of autophagy status and its relationship with immunity and inflammation in ischemic stroke through integrated transcriptomic and single-cell sequencing
Genes & Immunity (2025)
-
Chemically engineered antibodies for autophagy-based receptor degradation
Nature Chemical Biology (2025)
-
RNF128 deficiency in macrophages promotes colonic inflammation by suppressing the autophagic degradation of S100A8
Cell Death & Disease (2025)
-
The interconnective role of the UPS and autophagy in the quality control of cancer mitochondria
Cellular and Molecular Life Sciences (2025)