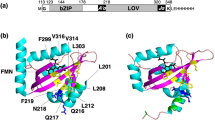
Abstract
Light controls important physiological and morphological responses in fungi. Fungi can sense near-ultraviolet, blue, green, red and far-red light using up to 11 photoreceptors and signalling cascades to control a large proportion of the genome and thereby adapt to environmental conditions. The blue-light photoreceptor functions directly as a transcriptional regulator in the nucleus, whereas the red-light-sensing and far-red-light-sensing phytochrome induces a signalling pathway to transduce the signal from the cytoplasm to the nucleus. Green light can be sensed by retinal-binding proteins, known as opsins, but the signalling mechanisms are not well understood. In this Review, we discuss light signalling processes in fungi, their signalling cascades and recent insights into the integration of light signalling pathways with other regulatory circuits in fungal cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Fuller, K. K., Loros, J. J. & Dunlap, J. C. Fungal photobiology: visible light as a signal for stress, space and time. Curr. Genet. 61, 275–288 (2015).
Dasgupta, A., Fuller, K. K., Dunlap, J. C. & Loros, J. J. Seeing the world differently: variability in the photosensory mechanisms of two model fungi. Environ. Microbiol. 18, 5–20 (2015).
Fischer, R., Aguirre, J., Herrera-Estrella, A. & Corrochano, L. M. The complexity of fungal vision. Microbiol. Spectr. https://doi.org/10.1128/microbiolspec.FUNK-0020-2016 (2016).
Schumacher, J. How light affects the life of Botrytis. Fungal Genet. Biol. 106, 26–41 (2017).
Schmoll, M. Light, stress, sex and carbon – the photoreceptor ENVOY as a central checkpoint in the physiology of Trichoderma reesei. Fungal Biol. 122, 479–486 (2018).
Riquelme, M. et al. Fungal morphogenesis: from the polarized growth of hyphae to complex reproduction and infection structures. Microbiol. Mol. Biol. Rev. 82, e00068–17 (2018).
Ruger-Herreros, C. et al. Regulation of conidiation by light in Aspergillus nidulans. Genetics 188, 809–822 (2011).
Chen, C. H., Ringelberg, C. S., Gross, R. H., Dunlap, J. C. & Loros, J. J. Genome-wide analysis of light-inducible responses reveals hierarchical light signalling in Neurospora. EMBO J. 28, 1029–1042 (2009).
Rosales-Saavedra, T. et al. Novel light-regulated genes in Trichoderma atroviride: a dissection by cDNA microarrays. Microbiology 152, 3305–3317 (2006).
Esquivel-Naranjo, E. U. et al. A Trichoderma atroviride stress-activated MAPK pathway integrates stress and light signals. Mol. Microbiol. 100, 860–876 (2016).
Yu, Z., Armant, O. & Fischer, R. Fungi use the SakA (HogA) pathway for phytochrome-dependent light signaling. Nat. Microbiol. 1, 16019 (2016). This paper presents the discovery of the link between red-light signalling and the HOG pathway.
Atoui, A. et al. Cross-talk between light and glucose regulation controls toxin production and morphogenesis in Aspergillus nidulans. Fungal Genet. Biol. 47, 962–972 (2010).
Fuller, K. K., Ringelberg, C. S., Loros, J. J. & Dunlap, J. C. The fungal pathogen Aspergillus fumigatus regulates growth, metabolism, and stress resistance in response to light. mBio 4, e00142–13 (2013).
Schmoll, M., Schuster, A., Silva Rdo, N. & Kubicek, C. P. The G-alpha protein GNA3 of Hypocrea jecorina (Anamorph Trichoderma reesei) regulates cellulase gene expression in the presence of light. Eukaryot. Cell 8, 410–420 (2009).
Bayram, O. et al. Changes of global gene expression and secondary metabolite accumulation during light-dependent Aspergillus nidulans development. Fungal Genet. Biol. 87, 30–53 (2016).
Silva, F., Torres-Martinez, S. & Garre, V. Distinct white collar-1 genes control specific light responses in Mucor circinelloides. Mol. Microbiol. 61, 1023–1037 (2006).
Mooney, J. L. & Yager, L. N. Light is required for conidiation in Aspergillus nidulans. Genes Dev. 4, 1473–1482 (1990).
Kües, U. et al. The A mating type and blue light regulate all known differentiation processes in the basidiomycete Coprinus cinereus. Mol. Gen. Genet. 260, 81–91 (1998).
Grünbacher, A. et al. Six hydrophobins are involved in hydrophobin rodlet formation in Aspergillus nidulans and contribute to hydrophobicity of the spore surface. PLOS ONE 9, e94546 (2014).
Röhrig, J., Kastner, C. & Fischer, R. Light inhibits spore germination through phytochrome in Aspergillus nidulans. Curr. Genet. 59, 55–62 (2013).
Duran, R., Cary, J. W. & Calvo, A. M. Role of the osmotic stress regulatory pathway in morphogenesis and secondary metabolism in fialmentous fungi. Toxins 2, 367–381 (2010).
Dunlap, J. C. & Loros, J. J. Making time: conservation of biological clocks from fungi to animals. Microbiol. Spectr. https://doi.org/10.1128/microbiolspec.FUNK-0039-2016 (2017).
Montenegro-Montero, A., Canessa, P. & Larrondo, L. F. Around the fungal clock: recent advances in the molecular study of circadian clocks in Neurospora and other fungi. Adv. Genet. 92, 107–184 (2015).
Lee, K., Loros, J. J. & Dunlap, J. C. Interconnected feedback loops in the Neurospora circadian system. Science 290, 277 (2000).
Arpaia, G., Loros, J. J., Dunlap, J. C., Morelli, G. & Macino, G. The interplay of light and the circadian clock. Independent dual regulation of clock-controlled gene ccg-2 (eas). Plant Physiol. 102, 1299–1305 (1993).
Cohen, R. & Delbrück, M. Photo-reactions in Phycomyces; growth and tropic responses to the stimulation of narrow test areas. J. Gen. Physiol. 42, 677–695 (1959).
Corrochano, L. M. & Galland, P. in The Mycota: Growth, differentiation and sexuality (ed. Wendland, J.) 235–256 (Springer, 2016).
O’Hara, A. & Jenkins, J. I. In vivo function of tryptophans in the Arabidopsis UV-B photoreceptor UVR8. Plant Cell 24, 3755–3766 (2012).
Losi, A. & Gärtner, W. The evolution of flavin-binding photoreceptors: an ancient chromophore serving trendy blue-light sensors. Annu. Rev. Plant Biol. 63, 49–72 (2012).
Pfeifer, A. et al. Time-resolved Fourier transform infrared study on photoadduct formation and secondary structural changes within the phototropin LOV domain. Biophys. J. 96, 1462–1470 (2009).
Crosson, S. & Moffat, K. Photoexcited structure of a plant photoreceptor domain reveals a light-driven molecular switch. Plant Cell 14, 1067–1075 (2002).
Heintzen, C., Loros, J. J. & Dunlap, J. C. The PAS protein VIVID defines a clock-associated feedback loop that represses light input, modulates gating, and regulates clock resetting. Cell 104, 453–464 (2001).
Zoltowski, B. D. et al. Conformational switching in the fungal light sensor Vivid. Science 316, 1054–1057 (2007). This is the first study to report the crystal structure of the LOV domain-containing protein VVD.
Dasgupta, A. et al. Biological significance of photoreceptor photocycle length: VIVID photocycle governs the dynamic VIVID-White Collar Complex pool mediating photo-adaptation and response to changes in light intensity. PLOS Genet. 11, e1005215 (2015).
Zoltowski, B. D., Vaccaro, B. & Crane, B. R. Mechanism-based tuning of a LOV domain photoreceptor. Nat. Chem. Biol. 5, 827–834 (2009).
Zoltowski, B. D. & Crane, B. R. Light activation of the LOV protein vivid generates a rapidly exchanging dimer. Biochemistry 47, 7012–7019 (2008).
Vaidya, A. T., Chen, C. H., Dunlap, J. C., Loros, J. J. & Crane, B. R. Structure of a light-activated LOV protein dimer that regulates transcription. Sci. Signal. 4, ra50 (2011).
Lee, C. T., Malzahn, E., Brunner, M. & Mayer, M. P. Light-induced differences in conformational dynamics of the circadian clock regulator VIVID. J. Mol. Biol. 426, 601–610 (2014).
Ganguly, A., Thiel, W. & Crane, B. R. Glutamine amide flip elicits long distance allosteric responses in the lov protein VIVID. J. Am. Chem. Soc. 139, 2972–2980 (2017).
Schwerdtfeger, C. & Linden, H. Blue light adaptation and desensitization of light signal transduction in Neurospora crassa. Mol. Microbiol. 39, 1080–1087 (2001).
Schwerdtfeger, C. & Linden, H. VIVID is a flavoprotein and serves as a fungal blue light photoreceptor for photoadaptation. EMBO J. 22, 4846–4855 (2003).
Ballario, P. et al. White collar-1, a central regulator of blue light responses in Neurospora crassa, is a zinc finger protein. EMBO J. 15, 1650–1657 (1996). This is the first study first to clone the photoreceptor gene.
Froehlich, A. C., Liu, Y., Loros, J. J. & Dunlap, J. C. White collar-1, a circadian blue light photoreceptor, binding to the frequency promoter. Science 297, 815–819 (2002).
He, Q. et al. White collar-1, a DNA binding transcription factor and a light sensor. Science 297, 840–843 (2002). References 43 and 44 show the breakthrough discovery of the chromophore attached to the photoreceptor, which is a transcription factor.
Cheng, P., He, Q., Yang, Y., Wang, L. & Liu, Y. Functional conservation of light, oxygen, or voltage domains in light sensing. Proc. Natl Acad. Sci. USA 100, 5938–5943 (2003).
Wang, B., Zhou, X., Loros, J. J. & Dunlap, J. C. Alternative use of DNA binding domains by the Neurospora white collar complex dictates circadian regulation and light responses. Mol. Cell. Biol. 36, 781–793 (2016).
Linden, H. & Macino, G. White collar 2, a partner in blue-light signal transduction, controlling expression of light-regulated genes in Neurospora crassa. EMBO J. 16, 98–109 (1997).
Schwerdtfeger, C. & Linden, H. Localization and light-dependent phosphorylation of white collar 1 and 2, the two central components of blue light signaling in Neurospora crassa. Eur. J. Biochem. 267, 414–421 (2000).
Cheng, P., Yang, Y., Wang, L., He, Q. & Liu, Y. WHITE COLLAR-1, a multifunctional Neurospora protein involved in the circadian feedback loops, light sensing, and transcription repression of wc-2. J. Biol. Chem. 278, 3801–3808 (2003).
Sanz, C. et al. Phycomyces MADB interacts with MADA to form the primary photoreceptor complex for fungal phototropism. Proc. Natl Acad. Sci. USA 106, 7095–7100 (2009).
Idnurm, A. et al. The Phycomyces madA gene encodes a blue-light photoreceptor for phototropism and other light responses. Proc. Natl Acad. Sci. USA 103, 4546–4551 (2006).
Sancar, A. Structure and function of DNA photolyase and cryptochrome blue light-photoreceptors. Chem. Rev. 103, 2203–2237 (2003).
Bayram, Ö., Biesemann, C., Krappmann, S., Galland, P. & Braus, G. H. More than a repair enzyme: Aspergillus nidulans photolyase-like CryA is a regulator of sexual development. Mol. Biol. Cell 19, 3254–3262 (2008).
Berrocal-Tito, G. M., Esquivel-Naranjo, E. U., Horwitz, B. A. & Herrera-Estrella, A. Trichoderma atroviride PHR1, a fungal photolyase responsible for DNA repair, autoregulates its own photoinduction. Eukaryot. Cell 6, 1682–1692 (2007).
Cohrs, K. C. & Schumacher, J. The two cryptochrome/photolyase family proteins fulfill distinct roles in DNA photorepair and regulation of conidiation in the gray mold fungus Botrytis cinerea. Appl. Environ. Microbiol. 83, e00812–17 (2017).
Brych, A. et al. White collar 1-induced photolyase expression contributes to UV-tolerance of Ustilago maydis. MicrobiologyOpen 5, 224–243 (2016).
Scheerer, P. et al. Crystal structure of opsin in its G-protein-interacting conformation. Nature 455, 497–502 (2008).
Spudich, J. L. The multitalented microbial sensory rhodopsins. Trends Microbiol. 14, 480–487 (2006).
Bieszke, J. A. et al. The nop-1 gene of Neurospora crassa encodes a seven transmembrane helix retinal-binding protein homologous to archaeal rhodopsins. Proc. Natl Acad. Sci. USA 96, 8034–8039 (1999).
Bieszke, J. A., Spudich, E. N., Scott, K. L., Borkovich, K. A. & Spudich, J. L. A eukaryotic protein, NOP-1, binds retinal to form an archaeal rhodopsin-like photochemically reactive pigment. Biochemistry 38, 14138–14145 (1999).
Ernst, O. P. et al. Microbial and animal rhodopsins: structures, functions and molecular mechanisms. Chem. Rev. 114, 126–163 (2014).
Waschuk, S. A., Bezerra, A. G. J., Shi, L. & Brown, L. S. Leptosphaeria rhodopsin: bacteriorhodopsin-like proton pump from a eukaryote. Proc. Natl Acad. Sci. USA 102, 6879–6883 (2005).
Brown, L. S., Dioumaev, A. K., Lanyi, J. K., Spudich, E. N. & Spudich, J. L. Photochemical reaction cycle and proton transfer in Neurospora rhodopsin. J. Biol. Chem. 276, 32495–32505 (2001).
Chow, B. Y. et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature 463, 98–102 (2010).
Rodriguez-Romero, J., Hedtke, M., Kastner, C., Müller, S. & Fischer, R. Fungi, hidden in soil or up in the air: light makes a difference. Annu. Rev. Microbiol. 64, 585–510 (2010).
Smith, H. Phytochromes and light signal perception by plants — an emerging synthesis. Nature 407, 585–591 (2000).
Inoue, K., Nishihama, R. & Kohchi, T. Evolutionary origin of phytochrome responses and signaling in land plants. Plant Cell Environ. 40, 2502–2508 (2017).
Rockwell, N. C., Su, Y. S. & Lagarias, J. C. Phytochrome structure and signaling mechanisms. Annu. Rev. Plant Biol. 57, 837–858 (2006).
Blumenstein, A. et al. The Aspergillus nidulans phytochrome FphA represses sexual development in red light. Curr. Biol. 15, 1833–1838 (2005).
Froehlich, A. C., Noh, B., Vierstra, R. D., Loros, J. & Dunlap, J. C. Genetic and molecular analysis of phytochromes from the filamentous fungus Neurospora crassa. Eukaryot. Cell 4, 2140–2152 (2005). References 69 and 70 are the first to characterize the fungal phytochromes.
Buchberger, T. & Lamparter, T. Streptophyte phytochromes exhibit an N-terminus of cyanobacterial origin and a C-terminus of proteobacterial origin. BMC Res. Notes 8, 1–13 (2015).
Li, F. W. et al. Phytochrome diversity in green plants and the origin of canonical plant phytochromes. Nat. Commun. 6, 7852 (2015).
Wagner, J. R., Brunzelle, J. S., Forest, K. T. & Vierstra, R. D. A light-sensing knot revealed by the structure of the chromophore-binding domain of phytochrome. Nature 438, 325–331 (2005). This is the first study to report the crystal structure of the photosensory domain of a phytochrome.
Brandt, S., von Stetten, D., Günther, M., Hildebrandt, P. & Frankenberg-Dinkel, N. The fungal phytochrome FphA from Aspergillus nidulans. J. Biol. Chem. 283, 34605–34614 (2008).
Adams, T. H., Wieser, J. K. & Yu, J.-H. Asexual sporulation in Aspergillus nidulans. Microbiol. Mol. Biol. Rev. 62, 35–54 (1998).
Smith, K. M. et al. Transcription factors in light and circadian clock signaling networks revealed by genomewide mapping of direct targets for Neurospora white collar complex. Eukaryot. Cell 9, 1549–1556 (2010).
Wu, C. et al. Genome-wide characterization of light-regulated genes in Neurospora crassa. G3 (Bethesda) 4, 1731–1745 (2014).
Sancar, C. et al. Combinatorial control of light induced chromatin remodeling and gene activation in Neurospora. PLOS Genet. 11, e1005105 (2015).
He, Q. & Liu, Y. Molecular mechanism of light responses in Neurospora: from light-induced transcription to photoadaptation. Genes Dev. 19, 2888–2899 (2005).
Schafmeier, T., Kaldi, K., Diernfellner, A., Mohr, C. & Brunner, M. Phosphorylation-dependent maturation of Neurospora circadian clock protein from a nuclear repressor toword a cytoplasmic activator. Genes Dev. 20, 297–306 (2005).
Cha, J., Chang, S. S., Huang, G., Cheng, P. & Liu, Y. Control of WHITE COLLAR localization by phosphorylation is a critical step in the circadian negative feedback process. EMBO J. 27, 3246–3255 (2008). This study reports the detailed mechanism of light activation through the WCC.
Talora, C., Franchi, L., Linden, H., Ballario, P. & Macino, G. Role of a white collar-1-white collar-2 complex in blue-light signal transduction. EMBO J. 18, 4961–4968 (1999).
He, Q. et al. CKI and CKII mediate the frequency-dependent phosphorylation of the WHITE COLLAR complex to close the Neurospora circadian negative feedback loop. Genes Dev. 20, 2552–2565 (2006).
Huang, G. et al. Protein kinase A and casein kinases mediate sequential phosphorylation events in the circadian negative feedback loop. Genes Dev. 21, 3283–3295 (2007).
Schafmeier, T. et al. Transcriptional feedback of Neurospora circadian clock gene by phosphorylation-dependent inactivation of its transcription factor. Cell 122, 235–246 (2005).
Cesbron, F., Oehler, M., Ha, N., Sancar, G. & Brunner, M. Transcriptional refractoriness is dependent on core promoter architecture. Nat. Commun. 6, 6753 (2015).
Li, C., Cesbron, F., Oehler, M., Brunner, M. & Höfer, T. Frequency modulation of transcriptional bursting enables sensitive and rapid gene regulation. Cell Syst. 25, 409–423.e11 (2018).
Brenna, A., Grimaldi, B., Filetici, P. & Ballario, P. Physical association of the WC-1 photoreceptor and the histone acetyltransferase NGF-1 is required for blue light signal transduction in Neurospora crassa. Mol. Biol. Cell 23, 3863–3872 (2012).
Grimaldi, B. et al. The Neurospora crassa White Collar-1 dependent bluit light response requires acetylation of histone H3 lysine 14 by NGF-1. Mol. Biol. Cell 17, 4576–4583 (2006).
Ruesch, C. E. et al. The histone H3 lysine 9 methyltransferase DIM-5 modifies chromatin at frequency and represses light-activated gene expression. G3 (Bethesda) 5, 93–101 (2014).
Raduwan, H., Isola, A. L. & Belden, W. J. Methylation of histone H3 on lysine 4 by the lysine methyltransferase SET1 protein is needed for normal clock gene expression. J. Biol. Chem. 288, 8380–8390 (2013).
Hurley, J. M. et al. Analysis of clock-regulated genes in Neurospora reveals widespread posttranscriptional control of metabolic potential. Proc. Natl Acad. Sci. USA 111, 16995–17002 (2014).
Purschwitz, J. et al. Functional and physical interaction of blue and red-light sensors in Aspergillus nidulans. Curr. Biol. 18, 255–259 (2008).
Casas-Flores, S., Rios-Momberg, M., Bibbins, M., Ponce-Noyola, P. & Herrera-Estrella, A. BLR-1 and BLR-2, key regulatory elements of photoconidiation and mycelial growth in Trichoderma atroviride. Microbiology 150, 3561–3569 (2004).
Casas-Flores, S. et al. Cross talk between a fungal blue-light perception system and the cyclic AMP signaling pathway. Eukaryot. Cell 5, 499–506 (2006).
Sánchez-Arrguín, A., Pérez-Martínez, A. S. & Herrera-Estrella, A. Proteomic analysis of Trichoderma atroviride reveals independent roles for transcription factors BLR-1 and BLR-2 in light and darkness. Eukaryot. Cell 11, 30–41 (2012).
Lokhandwala, J. et al. Structural biochemistry of a fungal LOV domain photoreceptor reveals an evolutionarily conserved pathway integrating light and oxidative stress. Structure 23, 116–125 (2015). This study reports the link between light and stress.
Chen, C. H., DeMay, B. S., Gladfelter, A. S., Dunlap, J. C. & Loros, J. J. Physical interraction between VIVID and white collar complex regulates photoadaptation in Neurospora. Proc. Natl Acad. Sci. USA 107, 16715–16720 (2010).
Malzahn, E., Ciprianidis, S., Káldi, K., Schafmeier, T. & Brunner, M. Photoadaptation in Neurospora by competitive interaction of activating and inhibitory LOV domains. Cell 142, 762–772 (2010).
Ruger-Herreros, C., Gil-Sanchez Mdel, M., Sancar, G., Brunner, M. & Corrochano, L. M. Alteration of light-dependent gene regulation by the absence of the RCO-1/RCM-1 repressor complex in the fungus Neurospora crassa. PLOS ONE 9, e95069 (2014).
Schumacher, J., Simon, A., Cohrs, K. C., Viaud, M. & Tudzynski, P. The transcription factor BcLTF1 regulates virulence and light responses in the necrotrophic plant pathogen Botrytis cinerea. PLOS Genet. 10, e1004040 (2014).
Corrochano, L. M. et al. Expansion of signal transduction pathways in fungi by extensive genome duplication. Curr. Biol. 26, 1577–1584 (2016).
Idnurm, A. & Heitman, J. Light controls growth and development via a conserved pathway in the fungal kingdom. PLOS Biol. 3, 615–626 (2005). This study clones the blue-light photoreceptor in Phycomyces blakesleeanus more than 30 years after the isolation of the corresponding mutant.
Schmoll, M., Franchi, L. & Kubicek, C. P. Envoy, a PAS/LOV domain protein of Hypocrea jecorina (anamorph Trichoderma reesei), modulates cellulase gene transcription in reponse to light. Eukaryot. Cell 4, 1998–2007 (2005).
Hedtke, M. et al. Light-dependent gene activation in Aspergillus nidulans is strictly dependent on phytochrome and involves the interplay of phytochrome and white collar-regulated histone H3 acetylation. Mol. Microbiol. 97, 733–745 (2015).
Cervantes-Badillo, M. G., Muñoz-Centeno, T., Uresti-Rivera, E. E., Argüello-Astorga, G. R. & Casas-Flores, S. The Trichoderma atroviride photolyase-encoding gene is transcriptionally regulated by non-canonical light response elements. FEBS J. 280, 3697–3708 (2013).
Olmedo, M., Ruger-Herreros, C., Luque, E. M. & Corrochano, L. M. A complex photoreceptor system mediates the regulation by light of the conidiation genes con-10 and con-6 in Neurospora crassa. Fungal Genet. Biol. 47, 352–363 (2010).
Wang, Z. et al. Light sensing by opsins and fungal ecology: NOP-1 modulates entry into sexual reproduction in response to environmental cues. Mol. Ecol. 27, 216–232 (2017).
García-Martínez, J., B., M., Avalos, J. & Termpitz, U. The CarO rhodopsin of the fungus Fusarium fujikuroi is a light-driven protein pump that retards spore germination. Sci. Rep. 5, 7798 (2015). This paper provides the first demonstration of fast proton-pumping activity of a fungal opsin.
Peñalva, M. A., Tilburn, J., Bignell, E. & Arst, H. N. J. Ambient pH gene regulation in fungi: making connections. Trends Microbiol. 16, 291–300 (2008).
Alkan, N. et al. Global aspects of pacC regulation of pathogenicity genes in Colletotrichum gloeosporioides as revealed by transcriptome analysis. Mol. Plant Microbe Interact. 26, 1345–1358 (2013).
Avelar, G. M. et al. A rhodopsin-guanylyl cyclase gene fusion functions in visual perception in a fungus. Curr. Biol. 24, 1234–1240 (2014).
Avelar, G. M. et al. A cyclic GMP-dependent K+ channel in the blastocladiomycete fungus Blastocladiella emersonii. Eukaryot. Cell 14, 958–963 (2015).
Bhoo, S.-H., Davis, S. J., Walker, J., Karniol, B. & Vierstra, R. D. Bacteriophytochromes are photochromic histidine kinases using a biliverdin chromophore. Nature 414, 776–779 (2001).
Nagatani, K. Plant biology. Lighting up the nucleus. Science 288, 821–822 (2000).
Bayram, O. et al. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science 320, 1504–1506 (2008).
Bayram, Ö. & Braus, G. H. Coordination of secondary metabolism and development in fungi: the velvet family of regulatory proteins. FEMS Microbiol. Rev. 36, 1–24 (2012).
Azuma, N. et al. In vitro analysis of His-Asp phosphorelays in Aspergillus nidulans: the first direct biochemical evidence for the existence of His-Asp phosphotransfer system in filamentous fungi. Biosci. Biotechnol. Biochem. 71, 2493–2502 (2007).
Wang, Z. et al. The fast-evolving phy-2 gene modulates sexual development in response to light in the model fungus Neurospora crassa. mBio https://doi.org/10.1128/mBio.02148-15 (2016).
Fuller, K. K., Cramer, R. A., Zegans, M. E., Dunlap, J. C. & Loros, J. J. Aspergillus fumigatus photobiology illuminates the marked heterogeneity between isolates. mBio 7, e01517-16 (2016).
Lukens, R. J. Reversal by red light of blue light inhibition of sporulation in Alternaria solani. Phytophathology 55, 1032 (1965).
Pruß, S. et al. Role of the Alternaria alternata blue-light receptor WC-1 (LreA) in spore formation and secondary metabolism. Appl. Environ. Microbiol. 80, 2582–2591 (2014).
García-Esquivel, M., Esquivel-Naranjo, E. U., Hernández-Oñate, M. A., Ibarra-Laclette, E. & Herrera-Estrella, A. The Trichoderma atroviride cryptochrome/photolyase genes regulate the expression of blr1-independent genes both in red and blue light. Fungal Biol. 120, 500–512 (2016).
Verma, S. & Idnurm, A. The Uve1 endonuclease is regulated by the White Collar Complex to protect Cryptococcus neoformans from UV damage. PLOS Genet. 9, e1003769 (2013).
Rangel, D. E. N., Fernandes, E. K. K., Braga, G. U. L. & Roberts, D. W. Visible light during mycelial growth and conidiation of Metarhizium robertsii produces conidia with increased stress tolerance. FEMS Microbiol. Lett. 315, 81–86 (2011).
Hu, Y. et al. Disruption of a phytochrome-like histidine kinase gene by homologous recombination leads to a significant reduction in vegetative growth, sclerotia production, and the pathogenicity of Botrytis cinerea. Physiol. Mol. Plant Pathol. 85, 25–33 (2014).
Qiu, L., Wang, J. J., Chu, Z. J., Ying, S. H. & Feng, M. G. Phytochrome controls conidiation in response to red/far-red light and daylight length and regulates multistress tolerance in Beauveria bassiana. Environ. Microbiol. 16, 2316–2328 (2014).
Hérivaux, A. et al. Major sensing proteins in pathogenic fungi: the hybrid histidine kinase family. PLOS Genet. 12, e1005683 (2016).
de Castro, P. A. et al. ChIP-seq reveals a role for CrzA in the Aspergillus fumigatus high-osmolarity glycerol response (HOG) signalling pathway. Mol. Microbiol. 94, 655–674 (2014).
Takeshita, N. et al. Pulses of Ca2+ coordinate actin assembly and exocytosis for stepwise cell extension. Proc. Natl Acad. Sci. USA 114, 5701–5706 (2017).
Zhou, L. et al. Superresolution and pulse-chase imaging reveal the role of vesicle transport in polar growth of fungal cells. Sci. Adv. 4, e1701798 (2018).
Tisch, D., Kubicek, C. P. & Schmoll, M. New insights into the mechanism of light modulated signaling by heterotrimeric G-proteins: ENVOY acts on gna1 and gna3 and adjusts cAMP levels in Trichoderma reesei (Hypocrea jecorina). Fungal Genet. Biol. 48, 631–640 (2011).
Schmoll, M., Esquivel-Naranjo, E. U. & Herrera-Estrella, A. Trichoderma in the light of day — physiology and development. Fungal Genet. Biol. 47, 909–916 (2010).
Bodvard, K., Jörhov, A., Blomberg, A., Molin, M. & Käll, M. The yeast transcription factor Crz1 is activated by light in a Ca2+/calcineurin-dependent and PKA-independent manner. PLOS ONE 8, e53404 (2013).
Bodvard, K. et al. Light-sensing via hydrogen peroxide and a peroxiredoxin. Nat. Commun. 8, 14791 (2017).
Hao, N. & O’Shea, E. K. Signal-dependent dynamics of transcription factor translocation controls gene expression. Nat. Struct. Mol. Biol. 19, 31–39 (2011).
Purschwitz, J., Müller, S. & Fischer, R. Mapping the interaction sites of Aspergillus nidulans phytochrome FphA with the global regulator VeA and the white collar protein LreB. Mol. Genet. Genomics. 281, 35–42 (2009).
Rauscher, S., Pacher, S., Hedtke, M., Kniemeyer, O. & Fischer, R. A phosphorylation code of the Aspergillus nidulans global regulator VelvetA (VeA) determines specific functions. Mol. Microbiol. 99, 909–924 (2016).
Smith, H. Light quality, photoperception, and plant strategy. Annu. Rev. Plant Physiol. 33, 481–518 (1982).
Njimona, I., Yang, R. & Lamparter, T. Temperature effects on bacterial phytochrome. PLOS ONE 9, e109794 (2014).
Legris, M. et al. Phytochrome B integrates light and temperature signals in Arabidopsis. Science 354, 897–900 (2016).
Jung, J. H. et al. Phytochromes function as thermosensors in Arabidopsis. Science 354, 886–889 (2016).
Casal, J. J. & Qüesta, J. I. Light and temperature cues: multitasking receptors and transcriptional integrators. New Phytol. 217, 1029–1034 (2018).
Shen, W. L. et al. Function of rhodopsin in temperature discrimination in Drosophila. Science 331, 1333–1336 (2011).
Nakasone, Y., Ono, T.-A., Ishii, A., Masuda, S. & Terazima, M. Temperature-sensitive reaction of a photosensor protein YcgF: possibility of a role of temperature sensor. Biochemistry 49, 2288–2296 (2010).
Fujii, Y. et al. Phototropin perceives temperature based on the lifetime of its photoactivated state. Proc. Natl Acad. Sci. USA 114, 9206–9211 (2017).
Schafmeier, T. et al. Circadian activity and abundance rhythms of the Neurospora clock tanscription factor WCC associated with rapid nucleo-cytoplamic shuttling. Genes Dev. 22, 3397–3402 (2008).
Hunt, S. M., Thompson, S., Elvin, M. & Heintzen, C. VIVID interacts with the WHITE COLLAR complex and FREQUENCY-interacting RNA helicase to alter light and clock responses in Neurospora. Proc. Natl Acad. Sci. USA 107, 16709–16714 (2010).
Acknowledgements
The work of the authors’ laboratory was supported by the German Science Foundation (DFG Fi 459/19-1) and the China Scholar Council (CSC). The authors thank A. Diernfellner (Heidelberg) and J. Schumacher (Münster) for Neurospora crassa strains and providing some images for Fig. 2.
Reviewer information
Nature Reviews Microbiology thanks A. Herrera-Estrella, J. Loros and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
Z.Y. and R.F. researched data for the article, made substantial contributions to discussions of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Chromophore
-
An organic molecule that absorbs light in the visible spectrum. Photoreceptors are proteins containing a chromophore, which upon light absorption cause structural changes of the attached protein.
- Flavin
-
An organic molecule with a characteristic tricyclic heterocycle (isoalloxazine).
- Linear tetrapyrrole
-
An organic molecule composed of four five-atom rings. They can be cyclic, as in haemoglobin, or linear, like in biliverdin.
- Phytochrome
-
A protein that contains a linear tetrapyrrole as a chromophore and absorbs red and far-red light. It is the main photosensor of plants and controls morphogenesis.
- Circadian clock
-
Describes a stable oscillation synchronized by light. The period is approximately (circa) a day (diem).
- Sporangiophore
-
The morphological structure of fungi that produce (resistant) spores, which are small entities for dispersal or for survival.
- Cryptochrome
-
A flavin-binding protein that senses blue light and is involved in circadian rhythms in higher eukaryotes.
- Photolyase
-
An enzyme that repairs DNA damage caused by ultraviolet light. The enzyme contains flavin as cofactor and itself requires blue light for activity.
- Photoadduct
-
A chromophore that attaches covalently to a protein.
- Photoadaptation
-
The phenomenon in which after light signalling the system becomes insensitive for a certain time.
- Rhodopsins
-
The photosensors of the retina. Rhodopsins contain retinal as a chromophore. Rhodopsins are also found in lower eukaryotes and in archaea.
- FRQ
-
A negative regulator of the circadian clock in fungi. Oscillation of transcription of clock-controlled genes requires the positive element, white collar 1 (WC-1), and the negative FRQ protein.
- Cleistothecia
-
The fruiting body of ascomycetes. Spores are produced after meiosis.
- Conidiation
-
The process of the formation of vegetative (asexual) spores. Usually special morphological structures, the conidiophores, are produced, which generate the conidia.
- Sclerotia
-
A multicellular structure used for survival of some fungi. In Botrytis cinerea, it is the prerequisite for sexual fruiting body (apothecia) formation.
- High osmolarity glycerol pathway
-
(HOG pathway). A signalling pathway that is required for the adaptation of yeast and other organisms to high osmolarity conditions.
Rights and permissions
About this article
Cite this article
Yu, Z., Fischer, R. Light sensing and responses in fungi. Nat Rev Microbiol 17, 25–36 (2019). https://doi.org/10.1038/s41579-018-0109-x
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41579-018-0109-x