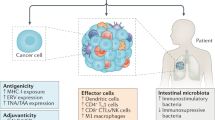
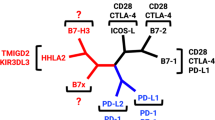
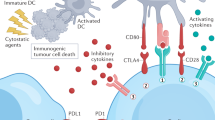
Abstract
Cell cycle proteins that are often dysregulated in malignant cells, such as cyclin-dependent kinase 4 (CDK4) and CDK6, have attracted considerable interest as potential targets for cancer therapy. In this context, multiple inhibitors of CDK4 and CDK6 have been developed, including three small molecules (palbociclib, abemaciclib and ribociclib) that are currently approved for the treatment of patients with breast cancer and are being extensively tested in individuals with other solid and haematological malignancies. Accumulating preclinical and clinical evidence indicates that the anticancer activity of CDK4/CDK6 inhibitors results not only from their ability to block the cell cycle in malignant cells but also from a range of immunostimulatory effects. In this Review, we discuss the ability of anticancer cell cycle inhibitors to modulate various immune functions in support of effective antitumour immunity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Woods, D. & Turchi, J. J. Chemotherapy induced DNA damage response: convergence of drugs and pathways. Cancer Biol. Ther. 14, 379–389 (2013).
Schirrmacher, V. From chemotherapy to biological therapy: a review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 54, 407–419 (2019).
Kent, L. N. & Leone, G. The broken cycle: E2F dysfunction in cancer. Nat. Rev. Cancer 19, 326–338 (2019).
Fry, D. W. et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol. Cancer Ther. 3, 1427–1438 (2004).
O’Leary, B., Finn, R. S. & Turner, N. C. Treating cancer with selective CDK4/6 inhibitors. Nat. Rev. Clin. Oncol. 13, 417–430 (2016).
Baughn, L. B. et al. A novel orally active small molecule potently induces G1 arrest in primary myeloma cells and prevents tumor growth by specific inhibition of cyclin-dependent kinase 4/6. Cancer Res. 66, 7661–7667 (2006).
Leonard, J. P. et al. Selective CDK4/6 inhibition with tumor responses by PD0332991 in patients with mantle cell lymphoma. Blood 119, 4597–4607 (2012).
Harbour, J. W., Luo, R. X., Dei Santi, A., Postigo, A. A. & Dean, D. C. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell 98, 859–869 (1999). This work is the first demonstration that CDK4 and CDK6 support cell cycle progression by initiating the inactivating phosphorylation of RB1.
Condorelli, R. et al. Polyclonal RB1 mutations and acquired resistance to CDK 4/6 inhibitors in patients with metastatic breast cancer. Ann. Oncol. 29, 640–645 (2018).
O’Leary, B. et al. The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Cancer Discov. 8, 1390–1403 (2018).
Niu, Y., Xu, J. & Sun, T. Cyclin-dependent kinases 4/6 inhibitors in breast cancer: current status, resistance, and combination strategies. J. Cancer 10, 5504–5517 (2019).
Klein, M. E., Kovatcheva, M., Davis, L. E., Tap, W. D. & Koff, A. CDK4/6 inhibitors: the mechanism of action may not be as simple as once thought. Cancer Cell 34, 9–20 (2018).
Wang, H. et al. The metabolic function of cyclin D3–CDK6 kinase in cancer cell survival. Nature 546, 426–430 (2017).
Lopez-Mejia, I. C. et al. CDK4 phosphorylates AMPKα2 to inhibit its activity and repress fatty acid oxidation. Mol. Cell 68, 336–349.e6 (2017).
Martinez-Carreres, L. et al. CDK4 regulates lysosomal function and mTORC1 activation to promote cancer cell survival. Cancer Res. 79, 5245–5259 (2019).
Liu, T. et al. CDK4/6-dependent activation of DUB3 regulates cancer metastasis through SNAIL1. Nat. Commun. 8, 13923 (2017).
Ameratunga, M., Kipps, E., Okines, A. F. C. & Lopez, J. S. To cycle or fight — CDK4/6 inhibitors at the crossroads of anticancer immunity. Clin. Cancer Res. 25, 21–28 (2019).
Laphanuwat, P. & Jirawatnotai, S. Immunomodulatory roles of cell cycle regulators. Front. Cell Dev. Biol. 7, 23 (2019).
Rane, S. G. et al. Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in β-islet cell hyperplasia. Nat. Genet. 22, 44–52 (1999).
Cooper, A. B. et al. A unique function for cyclin D3 in early B cell development. Nat. Immunol. 7, 489–497 (2006).
Peled, J. U. et al. Requirement for cyclin D3 in germinal center formation and function. Cell Res. 20, 631–646 (2010).
Cato, M. H., Chintalapati, S. K., Yau, I. W., Omori, S. A. & Rickert, R. C. Cyclin D3 is selectively required for proliferative expansion of germinal center B cells. Mol. Cell Biol. 31, 127–137 (2011).
Sicinska, E. et al. Essential role for cyclin D3 in granulocyte colony-stimulating factor-driven expansion of neutrophil granulocytes. Mol. Cell Biol. 26, 8052–8060 (2006).
Kozar, K. et al. Mouse development and cell proliferation in the absence of D-cyclins. Cell 118, 477–491 (2004).
Malumbres, M. et al. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell 118, 493–504 (2004). Together with Kozar et al. (2004), this work reports the ability of some mammalian cells to progress in the cell cycle in the absence of both CDK4 and CDK6, or of all cyclin D isoforms.
Sicinska, E. et al. Requirement for cyclin D3 in lymphocyte development and T cell leukemias. Cancer Cell 4, 451–461 (2003).
Franklin, D. S. et al. CDK inhibitors p18INK4c and p27Kip1 mediate two separate pathways to collaboratively suppress pituitary tumorigenesis. Genes. Dev. 12, 2899–2911 (1998).
Tourigny, M. R. et al. CDK inhibitor p18INK4c is required for the generation of functional plasma cells. Immunity 17, 179–189 (2002).
Hu, M. G. et al. A requirement for cyclin-dependent kinase 6 in thymocyte development and tumorigenesis. Cancer Res. 69, 810–818 (2009).
Hu, M. G. et al. CDK6 kinase activity is required for thymocyte development. Blood 117, 6120–6131 (2011).
Sharpless, N. E. et al. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature 413, 86–91 (2001).
Solvason, N. et al. Cyclin D2 is essential for BCR-mediated proliferation and CD5 B cell development. Int. Immunol. 12, 631–638 (2000).
Goel, S. et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 548, 471–475 (2017). This work shows that CDK4/CDK6 inhibitors favour the expression of endogenous retroviral elements that drive type III interferon secretion and suppress the proliferation of Treg cells.
Konig, S. et al. First insight into the kinome of human regulatory T cells. PLoS One 7, e40896 (2012). This study uses a kinase-selective proteomic strategy to compare TCR signalling in effector T cells and Treg cells, leading to the identification of CDK6 as an effector of TCR-driven proliferation in Treg cells.
Rowell, E. A., Wang, L., Chunder, N., Hancock, W. W. & Wells, A. D. Regulation of T cell differentiation and alloimmunity by the cyclin-dependent kinase inhibitor p18ink4c. PLoS One 9, e91587 (2014).
Wells, A. D. & Morawski, P. A. New roles for cyclin-dependent kinases in T cell biology: linking cell division and differentiation. Nat. Rev. Immunol. 14, 261–270 (2014).
Malumbres, M. Cyclin-dependent kinases. Genome Biol. 15, 122 (2014).
Boussiotis, V. A. et al. p27kip1 functions as an anergy factor inhibiting interleukin 2 transcription and clonal expansion of alloreactive human and mouse helper T lymphocytes. Nat. Med. 6, 290–297 (2000).
Jatzek, A., Marie Tejera, M., Plisch, E. H., Fero, M. L. & Suresh, M. T-cell intrinsic and extrinsic mechanisms of p27Kip1 in the regulation of CD8 T-cell memory. Immunol. Cell Biol. 91, 120–129 (2013).
Pareek, T. K. et al. Cyclin-dependent kinase 5 activity is required for T cell activation and induction of experimental autoimmune encephalomyelitis. J. Exp. Med. 207, 2507–2519 (2010).
Chunder, N., Wang, L., Chen, C., Hancock, W. W. & Wells, A. D. Cyclin-dependent kinase 2 controls peripheral immune tolerance. J. Immunol. 189, 5659–5666 (2012).
Tsai, L. H., Delalle, I., Caviness, V. S. Jr., Chae, T. & Harlow, E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature 371, 419–423 (1994).
Rowell, E. A., Wang, L., Hancock, W. W. & Wells, A. D. The cyclin-dependent kinase inhibitor p27kip1 is required for transplantation tolerance induced by costimulatory blockade. J. Immunol. 177, 5169–5176 (2006).
Askew, D. et al. Cyclin-dependent kinase 5 activity is required for allogeneic T-cell responses after hematopoietic cell transplantation in mice. Blood 129, 246–256 (2017).
Cortes, N., Guzman-Martinez, L., Andrade, V., Gonzalez, A. & Maccioni, R. B. CDK5: a unique CDK and its multiple roles in the nervous system. J. Alzheimers Dis. 68, 843–855 (2019).
Lam, E., Choi, S. H., Pareek, T. K., Kim, B. G. & Letterio, J. J. Cyclin-dependent kinase 5 represses Foxp3 gene expression and Treg development through specific phosphorylation of Stat3 at Serine 727. Mol. Immunol. 67, 317–324 (2015).
Lam, E., Pareek, T. K. & Letterio, J. J. Cdk5 controls IL-2 gene expression via repression of the mSin3a–HDAC complex. Cell Cycle 14, 1327–1336 (2015).
Morawski, P. A., Mehra, P., Chen, C., Bhatti, T. & Wells, A. D. Foxp3 protein stability is regulated by cyclin-dependent kinase 2. J. Biol. Chem. 288, 24494–24502 (2013).
Vanden Bush, T. J. & Bishop, G. A. CDK-mediated regulation of cell functions via c-Jun phosphorylation and AP-1 activation. PLoS One 6, e19468 (2011).
Amulic, B. et al. Cell-cycle proteins control production of neutrophil extracellular traps. Dev. Cell 43, 449–462.e5 (2017).
Dannappel, M. V., Sooraj, D., Loh, J. J. & Firestein, R. Molecular and in vivo functions of the CDK8 and CDK19 kinase modules. Front. Cell Dev. Biol. 6, 171 (2018).
Johannessen, L. et al. Small-molecule studies identify CDK8 as a regulator of IL-10 in myeloid cells. Nat. Chem. Biol. 13, 1102–1108 (2017).
Martinek, J., Wu, T. C., Cadena, D., Banchereau, J. & Palucka, K. Interplay between dendritic cells and cancer cells. Int. Rev. Cell Mol. Biol. 348, 179–215 (2019).
Wculek, S. K. et al. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 20, 7–24 (2020).
Yu, C. R., Young, H. A. & Ortaldo, J. R. Characterization of cytokine differential induction of STAT complexes in primary human T and NK cells. J. Leukoc. Biol. 64, 245–258 (1998).
Vivier, E. et al. Innate or adaptive immunity? The example of natural killer cells. Science 331, 44–49 (2011).
Bancerek, J. et al. CDK8 kinase phosphorylates transcription factor STAT1 to selectively regulate the interferon response. Immunity 38, 250–262 (2013).
Guo, Z., Wang, G., Lv, Y., Wan, Y. Y. & Zheng, J. Inhibition of Cdk8/Cdk19 activity promotes Treg cell differentiation and suppresses autoimmune diseases. Front. Immunol. 10, 1988 (2019).
Witalisz-Siepracka, A. et al. NK cell-specific CDK8 deletion enhances antitumor responses. Cancer Immunol. Res. 6, 458–466 (2018).
Alarcon, C. et al. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-β pathways. Cell 139, 757–769 (2009).
Anders, L. et al. A systematic screen for CDK4/6 substrates links FOXM1 phosphorylation to senescence suppression in cancer cells. Cancer Cell 20, 620–634 (2011).
Vijayaraghavan, S. et al. CDK4/6 and autophagy inhibitors synergistically induce senescence in Rb positive cytoplasmic cyclin E negative cancers. Nat. Commun. 8, 15916 (2017).
Yoshida, A., Lee, E. K. & Diehl, J. A. Induction of therapeutic senescence in vemurafenib-resistant melanoma by extended inhibition of CDK4/6. Cancer Res. 76, 2990–3002 (2016).
Kovatcheva, M. et al. ATRX is a regulator of therapy induced senescence in human cells. Nat. Commun. 8, 386 (2017).
Llanos, S. et al. Lysosomal trapping of palbociclib and its functional implications. Oncogene 38, 3886–3902 (2019).
Narita, M. et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113, 703–716 (2003).
Gorgoulis, V. et al. Cellular senescence: defining a path forward. Cell 179, 813–827 (2019). This recent review article provides a critical discussion of key cellular and molecular features of senescence from the International Cell Senescence Association.
Textor, S. et al. Human NK cells are alerted to induction of p53 in cancer cells by upregulation of the NKG2D ligands ULBP1 and ULBP2. Cancer Res. 71, 5998–6009 (2011).
Bauer, S. et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285, 727–729 (1999).
Lopez-Soto, A., Gonzalez, S., Smyth, M. J. & Galluzzi, L. Control of metastasis by NK cells. Cancer Cell 32, 135–154 (2017).
Coppe, J. P. et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 6, 2853–2868 (2008).
Faget, D. V., Ren, Q. & Stewart, S. A. Unmasking senescence: context-dependent effects of SASP in cancer. Nat. Rev. Cancer 19, 439–453 (2019).
Xue, W. et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445, 656–660 (2007).
Iannello, A., Thompson, T. W., Ardolino, M., Lowe, S. W. & Raulet, D. H. p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J. Exp. Med. 210, 2057–2069 (2013).
Kang, T. W. et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 479, 547–551 (2011).
Lujambio, A. et al. Non-cell-autonomous tumor suppression by p53. Cell 153, 449–460 (2013).
Guerriero, J. L. Macrophages: their untold story in T cell activation and function. Int. Rev. Cell Mol. Biol. 342, 73–93 (2019).
Vitale, I. et al. Mutational and antigenic landscape in tumor progression and cancer immunotherapy. Trends Cell Biol. 29, 396–416 (2019).
Demaria, M. et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 7, 165–176 (2017).
Galluzzi, L. & Vitale, I. Oncogene-induced senescence and tumour control in complex biological systems. Cell Death Differ. 25, 1005–1006 (2018).
Sun, L., Wu, J., Du, F., Chen, X. & Chen, Z. J. Cyclic GMP–AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791 (2013).
Vanpouille-Box, C., Hoffmann, J. A. & Galluzzi, L. Pharmacological modulation of nucleic acid sensors — therapeutic potential and persisting obstacles. Nat. Rev. Drug. Discov. 18, 845–867 (2019).
Acosta, J. C. et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133, 1006–1018 (2008).
Gluck, S. et al. Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat. Cell Biol. 19, 1061–1070 (2017).
Sabapathy, K. & Lane, D. P. Therapeutic targeting of p53: all mutants are equal, but some mutants are more equal than others. Nat. Rev. Clin. Oncol. 15, 13–30 (2018).
Vilgelm, A. E. et al. Connecting the dots: therapy-induced senescence and a tumor-suppressive immune microenvironment. J. Natl Cancer Inst. 108, djv406 (2016).
Mitchell, J. P. & Carmody, R. J. NF-κB and the transcriptional control of inflammation. Int. Rev. Cell Mol. Biol. 335, 41–84 (2018).
Jerby-Arnon, L. et al. A cancer cell program promotes T cell exclusion and resistance to checkpoint blockade. Cell 175, 984–997.e24 (2018).
Ruscetti, M. et al. NK cell-mediated cytotoxicity contributes to tumor control by a cytostatic drug combination. Science 362, 1416–1422 (2018). This report describes the ability of NK cells to support the eradication of lung cancer cells exposed to a MAPK inhibitor and a CDK4/CDK6 inhibitor, which reflects the ability of the latter to drive the secretion of immunostimulatory cytokines.
Schaer, D. A. et al. The CDK4/6 inhibitor abemaciclib induces a T cell inflamed tumor microenvironment and enhances the efficacy of PD-L1 checkpoint blockade. Cell Rep. 22, 2978–2994 (2018).
Zhang, H. et al. CDK7 inhibition potentiates genome instability triggering anti-tumor immunity in small cell lung cancer. Cancer Cell 37, 37–54.e9 (2020).
Zitvogel, L., Galluzzi, L., Kepp, O., Smyth, M. J. & Kroemer, G. Type I interferons in anticancer immunity. Nat. Rev. Immunol. 15, 405–414 (2015).
Ishak, C. A., Classon, M. & De Carvalho, D. D. Deregulation of retroelements as an emerging therapeutic opportunity in cancer. Trends Cancer 4, 583–597 (2018).
Sprooten, J., Agostinis, P. & Garg, A. D. Type I interferons and dendritic cells in cancer immunotherapy. Int. Rev. Cell Mol. Biol. 348, 217–262 (2019).
Kollmann, K. et al. A kinase-independent function of CDK6 links the cell cycle to tumor angiogenesis. Cancer Cell 24, 167–181 (2013).
Terme, M. et al. VEGFA–VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 73, 539–549 (2013).
Tanchot, C. et al. Tumor-infiltrating regulatory T cells: phenotype, role, mechanism of expansion in situ and clinical significance. Cancer Microenviron. 6, 147–157 (2013).
Obata, Y. et al. The epigenetic regulator Uhrf1 facilitates the proliferation and maturation of colonic regulatory T cells. Nat. Immunol. 15, 571–579 (2014).
De Simone, M. et al. Transcriptional landscape of human tissue lymphocytes unveils uniqueness of tumor-infiltrating T regulatory cells. Immunity 45, 1135–1147 (2016).
Heng, T. S., Painter, M. W. & Immunological Genome Project Consortium. The Immunological Genome Project: networks of gene expression in immune cells. Nat. Immunol. 9, 1091–1094 (2008).
Deng, J. et al. CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Discov. 8, 216–233 (2018). This paper reports the ability of CDK4/CDK6 inhibitors to derepress transcription factors of the NFAT family, thereby boosting T cell activation.
McCartney, A. et al. Mechanisms of resistance to CDK4/6 inhibitors: potential implications and biomarkers for clinical practice. Front. Oncol. 9, 666 (2019).
Galluzzi, L., Yamazaki, T. & Kroemer, G. Linking cellular stress responses to systemic homeostasis. Nat. Rev. Mol. Cell Biol. 19, 731–745 (2018).
Munoz-Espin, D. et al. A versatile drug delivery system targeting senescent cells. EMBO Mol. Med. 10 (2018).
Formenti, S. C. et al. Focal irradiation and systemic TGFβ blockade in metastatic breast cancer. Clin. Cancer Res. 24, 2493–2504 (2018).
Mariathasan, S. et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548 (2018).
Tauriello, D. V. F. et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554, 538–543 (2018).
Cornell, L., Wander, S. A., Visal, T., Wagle, N. & Shapiro, G. I. MicroRNA-mediated suppression of the TGF-β pathway confers transmissible and reversible CDK4/6 inhibitor resistance. Cell Rep. 26, 2667–2680.e7 (2019).
Kitamura, T. et al. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J. Exp. Med. 212, 1043–1059 (2015).
Qian, B. Z. et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475, 222–225 (2011).
Bonapace, L. et al. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature 515, 130–133 (2014).
Rybstein, M. D., Bravo-San Pedro, J. M., Kroemer, G. & Galluzzi, L. The autophagic network and cancer. Nat. Cell Biol. 20, 243–251 (2018).
Yoshida, A. et al. SLC36A1–mTORC1 signaling drives acquired resistance to CDK4/6 inhibitors. Sci. Adv. 5, eaax6352 (2019).
Michaloglou, C. et al. Combined inhibition of mTOR and CDK4/6 is required for optimal blockade of E2F function and long-term growth inhibition in estrogen receptor-positive breast cancer. Mol. Cancer Ther. 17, 908–920 (2018).
Galluzzi, L., Bravo-San Pedro, J. M., Levine, B., Green, D. R. & Kroemer, G. Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat. Rev. Drug. Discov. 16, 487–511 (2017).
Garcia-Prat, L. et al. Autophagy maintains stemness by preventing senescence. Nature 529, 37–42 (2016).
Laberge, R. M. et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat. Cell Biol. 17, 1049–1061 (2015).
Zhong, Z., Sanchez-Lopez, E. & Karin, M. Autophagy, inflammation, and immunity: a troika governing cancer and its treatment. Cell 166, 288–298 (2016).
Spranger, S. & Gajewski, T. F. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat. Rev. Cancer 18, 139–147 (2018).
Toso, A. et al. Enhancing chemotherapy efficacy in Pten-deficient prostate tumors by activating the senescence-associated antitumor immunity. Cell Rep. 9, 75–89 (2014).
Di Mitri, D. et al. Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature 515, 134–137 (2014).
Vilgelm, A. E. et al. MDM2 antagonists overcome intrinsic resistance to CDK4/6 inhibition by inducing p21. Sci. Transl Med. 11, eaav7171 (2019).
Ruhland, M. K. et al. Stromal senescence establishes an immunosuppressive microenvironment that drives tumorigenesis. Nat. Commun. 7, 11762 (2016).
Guan, X. et al. Stromal senescence by prolonged CDK4/6 inhibition potentiates tumor growth. Mol. Cancer Res. 15, 237–249 (2017).
Laroche-Clary, A. et al. Combined targeting of MDM2 and CDK4 is synergistic in dedifferentiated liposarcomas. J. Hematol. Oncol. 10, 123 (2017).
Zhang, J. et al. Cyclin D–CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance. Nature 553, 91–95 (2018). This work is the first demonstration that CDK4 promotes proteasomal degradation of PDL1, which suggests that immune checkpoint blockers specific for PD1 or PDL1 could be potential therapeutic partners for CDK4/CDK6 inhibitors.
Galluzzi, L., Chan, T. A., Kroemer, G., Wolchok, J. D. & Lopez-Soto, A. The hallmarks of successful anticancer immunotherapy. Sci. Transl Med. 10, eaat7807 (2018).
Jin, X. et al. Phosphorylated RB promotes cancer immunity by inhibiting NF-κB activation and PD-L1 expression. Mol. Cell 73, 22–35 e26 (2019). This study documents the ability of phosphorylated RB1 to physically interact with and hence inhibit NF-κB, resulting in suppression of CD274 transcription.
Valenzuela, C. A. et al. SASP-dependent interactions between senescent cells and platelets modulate migration and invasion of cancer cells. Int. J. Mol. Sci. 20, E5292 (2019).
Kanikarla-Marie, P. et al. Platelet metabolism and other targeted drugs; potential impact on immunotherapy. Front. Oncol. 8, 107 (2018).
Rolfes, V. et al. PD-L1 is expressed on human platelets and is affected by immune checkpoint therapy. Oncotarget 9, 27460–27470 (2018).
Cingoz, O. & Goff, S. P. Cyclin-dependent kinase activity is required for type I interferon production. Proc. Natl Acad. Sci. USA 115, E2950–E2959 (2018).
Buque, A. & Galluzzi, L. Modeling tumor immunology and immunotherapy in mice. Trends Cancer 4, 599–601 (2018).
Acevedo, M. et al. A CDK4/6-dependent epigenetic mechanism protects cancer cells from PML-induced senescence. Cancer Res. 76, 3252–3264 (2016).
Baksh, S. et al. NFATc2-mediated repression of cyclin-dependent kinase 4 expression. Mol. Cell 10, 1071–1081 (2002).
Gelbert, L. M. et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest. N. Drugs 32, 825–837 (2014).
Chen, P. et al. Spectrum and degree of CDK drug interactions predicts clinical performance. Mol. Cancer Ther. 15, 2273–2281 (2016).
Toogood, P. L. et al. Discovery of a potent and selective inhibitor of cyclin-dependent kinase 4/6. J. Med. Chem. 48, 2388–2406 (2005).
Hsieh, F. S. et al. Palbociclib induces activation of AMPK and inhibits hepatocellular carcinoma in a CDK4/6-independent manner. Mol. Oncol. 11, 1035–1049 (2017).
Bates, S. et al. CDK6 (PLSTIRE) and CDK4 (PSK-J3) are a distinct subset of the cyclin-dependent kinases that associate with cyclin D1. Oncogene 9, 71–79 (1994).
Tsai, L. H., Harlow, E. & Meyerson, M. Isolation of the human cdk2 gene that encodes the cyclin A- and adenovirus E1A-associated p33 kinase. Nature 353, 174–177 (1991).
Koff, A. et al. Human cyclin E, a new cyclin that interacts with two members of the CDC2 gene family. Cell 66, 1217–1228 (1991).
Kato, J., Matsushime, H., Hiebert, S. W., Ewen, M. E. & Sherr, C. J. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes. Dev. 7, 331–342 (1993).
van den Heuvel, S. & Harlow, E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science 262, 2050–2054 (1993).
Fang, F. & Newport, J. W. Evidence that the G1–S and G2–M transitions are controlled by different cdc2 proteins in higher eukaryotes. Cell 66, 731–742 (1991).
King, R. W. et al. A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell 81, 279–288 (1995).
Quelle, D. E. et al. Cloning and characterization of murine p16INK4a and p15INK4b genes. Oncogene 11, 635–645 (1995).
Serrano, M., Hannon, G. J. & Beach, D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 366, 704–707 (1993).
Guan, K. L. et al. Growth suppression by p18, a p16INK4/MTS1- and p14INK4B/MTS2-related CDK6 inhibitor, correlates with wild-type pRb function. Genes. Dev. 8, 2939–2952 (1994).
Okuda, T. et al. Molecular cloning, expression pattern, and chromosomal localization of human CDKN2D/INK4d, an inhibitor of cyclin D-dependent kinases. Genomics 29, 623–630 (1995).
Harper, J. W., Adami, G. R., Wei, N., Keyomarsi, K. & Elledge, S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75, 805–816 (1993).
Polyak, K. et al. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 78, 59–66 (1994).
Lee, M. H., Reynisdottir, I. & Massague, J. Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes. Dev. 9, 639–649 (1995).
Galluzzi, L. et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 25, 486–541 (2018).
Wang, E. Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved. Cancer Res. 55, 2284–2292 (1995).
Zhu, Y. et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15, 428–435 (2016).
Di Leonardo, A., Linke, S. P., Clarkin, K. & Wahl, G. M. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes. Dev. 8, 2540–2551 (1994).
Alcorta, D. A. et al. Involvement of the cyclin-dependent kinase inhibitor p16INK4a in replicative senescence of normal human fibroblasts. Proc. Natl Acad. Sci. USA 93, 13742–13747 (1996).
Acknowledgements
G.P., S.C.F. and L.G. are supported by the 2019 Laura Ziskin Prize in Translational Research (no. ZP-6177) from the Stand Up to Cancer initiative. S.C.-K. and L.G. are supported by a Mantle Cell Lymphoma Research Initiative grant from the Leukemia and Lymphoma Society. S.C.-K. is further supported by a P01 grant (no. CA214274) from the National Institutes of Health/National Cancer Institute. L.G. is further supported by a Breakthrough Level 2 grant from the US Department of Defense, Breast Cancer Research Program (no. BC180476P1), a start-up grant from the Department of Radiation Oncology at Weill Cornell Medicine (New York, USA), a Rapid Response Grant from the Functional Genomics Initiative (New York, USA), industrial collaborations with Lytix (Oslo, Norway) and Phosplatin (New York, USA), and donations from Phosplatin (New York, USA), the Luke Heller TECPR2 Foundation (Boston, USA) and Sotio a.s. (Prague, Czech Republic).
Author information
Authors and Affiliations
Contributions
G.P. and L.G. conceived the idea for the Review and wrote the first version of the manuscript, with constructive input from S.C.F. and S.C.-K. G.P. prepared display items under the supervision of L.G. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
L.G. received consulting fees from OmniSEQ, Astra Zeneca, Inzen and the Luke Heller TECPR2 Foundation, and is a member of the Scientific Advisory Committee of Boehringer Ingelheim, The Longevity Labs and OmniSEQ. G.P., S.C.F. and S.C.-K. declare no competing interests.
Additional information
Peer review information
Nature Reviews Immunology thanks A. Wells and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Related links
ClinicalTrials.gov: http://www.clinicaltrials.gov
Glossary
- Retinoblastoma 1
-
(RB1). A multifunctional tumour suppressor protein that is dysfunctional in most human cancers. Biallelic germline loss of RB1 is associated with hereditary paediatric retinoblastoma.
- Hormone receptor-positive breast cancer
-
A common form of breast cancer characterized by the expression of oestrogen receptor 1 and/or progesterone receptor.
- 13q14 deletion
-
A frequent genomic alteration, consisting of the deletion of a portion of the long arm of chromosome 13 that includes the retinoblastoma 1 (RB1) gene.
- Type 1 diabetes
-
An autoimmune form of glucose intolerance that originates from the immunological destruction of insulin-producing pancreatic β-cells and, hence, can be treated with insulin administration.
- Notch signalling
-
A highly conserved signal transduction pathway that regulates the differentiation of multiple cell types.
- Histone deacetylase
-
(HDAC). One of multiple enzymes that repress transcriptional programmes by removing functional acetyl groups from histones.
- Mediator complex
-
A tetrameric cyclin-dependent kinase 8 (CDK8)-containing or CDK19-containing complex that is involved in transcriptional regulation.
- Senescence surveillance
-
An immunological mechanism that ensures the natural killer cell-dependent and T cell-dependent elimination of potentially pathogenic senescent cells.
- Hepatic stellate cells
-
Liver-resident stromal cells that are largely found in proximity of the vasculature and contribute to hepatic fibrosis and carcinogenesis.
- The Cancer Genome Atlas
-
A large, publicly available patient data set containing mutational, transcriptional and pathophysiological data from multiple independent cohorts of patients with cancer.
- Mitogen-activated protein kinase
-
(MAPK). The generic name commonly used to indicate one or more members of a large serine/threonine protein kinase family involved in numerous cellular functions, including proliferation.
- Patient-derived xenografts
-
Patient-derived tumour samples implanted into immunologically compatible mice for investigational purposes.
- Endogenous retroviral elements
-
DNA sequences encoded (but normally not expressed) by the genome of multiple mammals (including humans and mice) that resemble modern retroviruses.
- Micronucleation
-
The accumulation of small nuclear structures containing one or a few chromosomes resulting from one or more rounds of defective mitosis.
- Viral mimicry
-
The state whereby mammalian cells that are not exposed to an exogenous viral infection activate one or more pathways involved in viral resistance, including antigen presentation and interferon signalling.
- Senolytic
-
An agent that specifically kills senescent cells.
- Autophagy
-
A conserved mechanism through which all eukaryotic cells can dispose of superfluous or potentially toxic cytosolic material via lysosomal degradation.
- Cytokine release syndrome
-
A life-threatening condition characterized by systemic inflammation that can arise as a complication of some diseases, or as an adverse effect of some immunotherapeutic agents.
Rights and permissions
About this article
Cite this article
Petroni, G., Formenti, S.C., Chen-Kiang, S. et al. Immunomodulation by anticancer cell cycle inhibitors. Nat Rev Immunol 20, 669–679 (2020). https://doi.org/10.1038/s41577-020-0300-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-020-0300-y
This article is cited by
-
Strategies to enhance the therapeutic efficacy of anti-PD-1 antibody, anti-PD-L1 antibody and anti-CTLA-4 antibody in cancer therapy
Journal of Translational Medicine (2024)
-
Pan-inhibition of the three H2S synthesizing enzymes restrains tumor progression and immunosuppression in breast cancer
Cancer Cell International (2024)
-
Integrating artificial intelligence in osteosarcoma prognosis: the prognostic significance of SERPINE2 and CPT1B biomarkers
Scientific Reports (2024)
-
Palbociclib and letrozole for hormone receptor-positive HER2-negative breast cancer with residual disease after neoadjuvant chemotherapy
npj Breast Cancer (2024)
-
Targeting immunogenic cell stress and death for cancer therapy
Nature Reviews Drug Discovery (2024)