Abstract
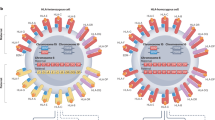
The prospect of transplanting cells and tissues without the risk of immune rejection or the need for powerful immunosuppressive drugs is the ‘holy grail’ of transplantation medicine. Now, with the advent of pluripotent stem cells, CRISPR–Cas9 and other gene-editing technologies, the race to create ‘off-the-shelf’ donor cells that are invisible to the immune system (‘universal cells’) has started. One important approach for creating such cells involves the manipulation of genes required for immune recognition, in particular HLA class I and II proteins. Other approaches leverage knowledge of immune-cloaking strategies used by certain bacteria, viruses, parasites, the fetus and cancer cells to induce tolerance to allogeneic cell-based therapies by modifying cells to express immune-suppressive molecules such as PD-L1 and CTLA4–Ig. Various academic groups as well as biotechnology and pharmaceutical companies are on the verge of bringing these therapies into the clinic.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Brent, L. A History of Transplantation Immunology. (Academic Press, San Diego, 1996).
Min, D. I. & Monaco, A. P. Complications associated with immunosuppressive therapy and their management. Pharmacotherapy 11, 119S–125S (1991).
Bix, M. et al. Rejection of class I MHC-deficient haemopoietic cells by irradiated MHC-matched mice. Nature 349, 329–331 (1991).
Liao, N. S., Bix, M., Zijlstra, M., Jaenisch, R. & Raulet, D. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science 253, 199–202 (1991).
Taylor, C. J. et al. Banking on human embryonic stem cells: estimating the number of donor cell lines needed for HLA matching. Lancet 366, 2019–2025 (2005).
Taylor, C. J., Peacock, S., Chaudhry, A. N., Bradley, J. A. & Bolton, E. M. Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell 11, 147–152 (2012).
Lanza, R. P., Cibelli, J. & West, M. D. Prospects for the use of nuclear transfer in human transplantation. Nat. Biotechnol. 17, 1171–1174 (1999).
Lanza, R. P., Cibelli, J. & West, M. D. Human therapeutic cloning. Nat. Med. 5, 975–977 (1999).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Nishikawa, S., Goldstein, R. A. & Nierras, C. R. The promise of human induced pluripotent stem cells for research and therapy. Nat. Rev. Mol. Cell Biol. 9, 725–729 (2008).
Mandai, M. et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. New. Engl. J. Med. 376, 1038–1046 (2017).
Schwartz, S. D. et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet 379, 713–720 (2012).
Schwartz, S. D. et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet 385, 509–516 (2015).
Frantz, S. Embryonic stem cell pioneer Geron exits field, cuts losses. Nat. Biotechnol. 30, 12–13 (2012).
ViaCyte, Inc. Center for beta cell therapy in diabetes and viacyte announce start of European clinical trial of human stem cell-derived implants in type 1 diabetes patients. PR Newswire https://www.prnewswire.com/news-releases/center-for-beta-cell-therapy-in-diabetes-and-viacyte-announce-start-of-european-clinical-trial-of-human-stem-cell-derived-implants-in-type-1-diabetes-patients-300781276.html (2019).
Miller, L. W. Trial of embryonic stem cell–derived cardiac progenitor cells. JACC 71, 439–442 (2018).
Cyranoski, D. ‘Reprogrammed’ stem cells implanted into patient with Parkinson’s disease. Nature https://doi.org/10.1038/d41586-018-07407-9 (2018).
Gornalusse, G. G. et al. HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat. Biotechnol. 35, 765–772 (2017).
Riolobos, L. et al. HLA engineering of human pluripotent stem cells. Mol. Ther. 21, 1232–1241 (2013).
Feng, Q. et al. Scalable generation of universal platelets from human pluripotent stem cells. Stem Cell Reports 3, 817–831 (2014).
Lu, P. et al. Generating hypoimmunogenic human embryonic stem cells by the disruption of beta 2-microglobulin. Stem Cell Rev. 9, 806–813 (2013).
Wang, D., Quan, Y., Yan, Q., Morales, J. E. & Wetsel, R. A. Targeted disruption of the β2-microglobulin gene minimizes the immunogenicity of human embryonic stem cells. Stem Cells Transl. Med. 4, 1234–1245 (2015).
Rong, Z. et al. An effective approach to prevent immune rejection of human ESC-derived allografts. Cell Stem Cell 14, 121–130 (2014).
Ostrander, E. A., Davis, B. W. & Ostrander, G. K. Transmissible tumors: breaking the cancer paradigm. Trends Genet. 32, 1–15 (2016).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Chao, M. P., Weissman, I. L. & Majeti, R. The CD47-SIRPalpha pathway in cancer immune evasion and potential therapeutic implications. Curr. Opin. Immunol. 24, 225–232 (2012).
Peter, M. E. et al. The role of CD95 and CD95 ligand in cancer. Cell Death Differ. 22, 885–886 (2015).
Yang, L., Pang, Y. & Moses, H. L. TGF-β and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 31, 220–227 (2010).
Harding, J. et al. Induction of long-term allogeneic cell acceptance and formation of immune privileged tissue in immunocompentent hosts. Preprint at bioRxiv https://www.biorxiv.org/content/10.1101/716571v1 (2019).
Deuse, T. et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol. 37, 252–258 (2019).
Petersdorf, E. W. Optimal HLA matching in hematopoietic cell transplantation. Curr. Opin. Immunol. 20, 588–593 (2008).
Zachary, A. A. & Leffell, M. S. HLA mismatching strategies for solid organ transplantation — a balancing act. Front Immunol. 7, 575 (2016).
Zijlstra, M. et al. β2-microglobulin deficient mice lack CD4–8+ cytolytic T cells. Nature 344, 742–746 (1990).
Koller, B. H., Marrack, P., Kappler, J. W. & Smithies, O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science 248, 1227–1230 (1990).
Zimmer, J. et al. Clinical and immunological aspects of HLA class I deficiency. QJM 98, 719–727 (2005).
Coffman, T. et al. Improved renal function in mouse kidney allografts lacking MHC class I antigens. J. Immunol. 151, 425–435 (1993).
Li, X. & Faustman, D. Use of donor β2-microglobulin-deficient transgenic mouse liver cells for isografts, allografts, and xenografts. Transplantation 55, 940–946 (1993).
Prange, S., Zucker, P., Jevnikar, A. M. & Singh, B. Transplanted MHC class I-deficient nonobese diabetic mouse islets are protected from autoimmune injury in diabetic nonobese recipients. Transplantation 71, 982–985 (2001).
Qian, S. et al. Impact of donor MHC class I or class II antigen deficiency on first- and second-set rejection of mouse heart or liver allografts. Immunology 88, 124–129 (1996).
Mattapally, S. et al. Human leukocyte antigen class I and II knockout human induced pluripotent stem cell-derived cells: universal donor for cell therapy. J. Am. Heart Assoc. 7, e010239 (2018).
Deuse, T. et al. Human leukocyte antigen I knockdown human embryonic stem cells induce host ignorance and achieve prolonged xenogeneic survival. Circulation 124, S3–S9 (2011).
Heath, W. R. & Carbone, F. R. Cross-presentation in viral immunity and self-tolerance. Nat. Rev. Immunol. 1, 126–134 (2001).
Benichou, G., Yamada, Y., Aoyama, A. & Madsen, J. C. Natural killer cells in rejection and tolerance of solid organ allografts. Curr. Opin. Organ. Transplant. 16, 47–53 (2011).
Lee, N. et al. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc. Natl Acad Sci. USA 95, 5199–5204 (1998).
Braud, V. M. et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 391, 795–799 (1998).
Navarro, F. et al. The ILT2(LIR1) and CD94/NKG2A NK cell receptors respectively recognize HLA-G1 and HLA-E molecules co-expressed on target cells. Eur. J. Immunol. 29, 277–283 (1999).
Pazmany, L. et al. Protection from natural killer cell-mediated lysis by HLA-G expression on target cells. Science 274, 792–795 (1996).
Gonen-Gross, T. et al. Inhibitory NK receptor recognition of HLA-G: regulation by contact residues and by cell specific expression at the fetal-maternal interface. PLOS ONE 5, e8941 (2010).
Rajagopalan, S. & Long, E. O. A human histocompatibility leukocyte antigen (HLA)-G-specific receptor expressed on all natural killer cells. J. Exp. Med. 189, 1093–1100 (1999).
Dulberger, C. L. et al. Human leukocyte antigen F presents peptides and regulates immunity through interactions with NK cell receptors. Immunity 46, 1018–1029.e7 (2017).
Zhao, H. X. et al. Enhanced immunological tolerance by HLA-G1 from neural progenitor cells (NPCS) derived from human embryonic stem cells (hESCs). Cell Physiol. Biochem. 44, 1435–1444 (2017).
Zhao, L., Teklemariam, T. & Hantash, B. M. Heterelogous expression of mutated HLA-G decreases immunogenicity of human embryonic stem cells and their epidermal derivatives. Stem Cell Res. 13, 342–354 (2014).
Horowitz, A. et al. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci. Transl Med. 5, 208ra145 (2013).
Hematti, P. Role of mesenchymal stromal cells in solid organ transplantation. Transplant. Rev. 22, 262–273 (2008).
Sugimoto, T. et al. Differential susceptibility of HLA class II antigens induced by gamma-interferon in human neuroblastoma cell lines. Cancer Res. 49, 1824–1828 (1989).
Soldevila, G. et al. HLA DR, DP, DQ induction in human islet beta cells by the cytokine combination IFN-γ+ TNF-α. Autoimmunity 6, 307–317 (1990).
DeSandro, A., Nagarajan, U. M. & Boss, J. M. The bare lymphocyte syndrome: molecular clues to the transcriptional regulation of major histocompatibility complex class II genes. Am. J. Hum. Genet. 65, 279–286 (1999).
Chang, C. H., Guerder, S., Hong, S. C., van Ewijk, W. & Flavell, R. A. Mice lacking the MHC class II transactivator (CIITA) show tissue-specific impairment of MHC class II expression. Immunity 4, 167–178 (1996).
Colunga, A., Hirata, R. & Russell, D. W. Generation of HLA Class II deficient human embryonic stem cells by AAV mediated knockout of RFXANK. Mol. Ther. 22, S14 (2014).
Chen, H. et al. Functional disruption of human leukocyte antigen II in human embryonic stem cell. Biol. Res. 48, 59 (2015).
Cosgrove, D. et al. Mice lacking MHC class II molecules. Cell 66, 1051–1066 (1991).
Ouederni, M. et al. Major histocompatibility complex class II expression deficiency caused by a RFXANK founder mutation: a survey of 35 patients. Blood 118, 5108–5218 (2011).
Grusby, M. J. et al. Mice lacking major histocompatibility complex class I and class II molecules. Proc. Natl Acad. Sci. USA 90, 3913–3917 (1993).
Kimbrel, E. A. & Lanza, R. Current status of pluripotent stem cells: moving the first therapies to the clinic. Nat. Rev. Drug Discov. 14, 681–692 (2015).
Di Lorenzo, T. P., Peakman, M. & Roep, B. O. Translational mini-review series on type 1 diabetes: systematic analysis of T cell epitopes in autoimmune diabetes. Clin. Exp. Immunol. 148, 1–16 (2007).
Mendell, J. R. et al. Dystrophin immunity in Duchenne’s muscular dystrophy. N. Engl. J. Med. 363, 1429–1437 (2010).
Cabrera, T. et al. High frequency of altered HLA class I phenotypes in invasive breast carcinomas. Hum. Immunol. 50, 127–134 (1996).
Koopman, L. A., Corver, W. E., van der Slik, A. R., Giphart, M. J. & Fleuren, G. J. Multiple genetic alterations cause frequent and heterogeneous human histocompatibility leukocyte antigen class I loss in cervical cancer. J. Exp. Med. 191, 961–976 (2000).
Cabrera, T. et al. High frequency of altered HLA class I phenotypes in laryngeal carcinomas. Hum. Immunol. 61, 499–506 (2000).
He, Y. et al. MHC class II expression in lung cancer. Lung Cancer 112, 75–80 (2017).
Heyman, M. et al. Interferon system defects in malignant T-cells. Leukemia 8, 425–434 (1994).
Kaplan, D. H. et al. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc. Natl Acad. Sci. USA 95, 7556–7561 (1998).
Cabrera, T. et al. High frequency of altered HLA class I phenotypes in invasive colorectal carcinomas. Tissue Antigens 52, 114–123 (1998).
van der Stoep, N., Biesta, P., Quinten, E. & van den Elsen, P. J. Lack of IFN-γ-mediated induction of the class II transactivator (CIITA) through promoter methylation is predominantly found in developmental tumor cell lines. Int. J. Cancer 97, 501–507 (2002).
van den Elsen, P. J., Holling, T. M., van der Stoep, N. & Boss, J. M. DNA methylation and expression of major histocompatibility complex class I and class II transactivator genes in human developmental tumor cells and in T cell malignancies. Clin. Immunol. 109, 46–52 (2003).
Croce, M. et al. Different levels of control prevent interferon-γ-inducible HLA-class II expression in human neuroblastoma cells. Oncogene 22, 7848–7857 (2003).
Satoh, A. et al. Epigenetic inactivation of class II transactivator (CIITA) is associated with the absence of interferon-γ-induced HLA-DR expression in colorectal and gastric cancer cells. Oncogene 23, 8876–8886 (2004).
Sucker, A. et al. Acquired IFNγ resistance impairs anti-tumor immunity and gives rise to T-cell-resistant melanoma lesions. Nat. Commun. 8, 15440 (2017).
Siddle, H. V. et al. Reversible epigenetic down-regulation of MHC molecules by devil facial tumour disease illustrates immune escape by a contagious cancer. Proc. Natl Acad. Sci. USA 110, 5103–5108 (2013).
Hsiao, Y. W., Liao, K. W., Hung, S. W. & Chu, R. M. Tumor-infiltrating lymphocyte secretion of IL-6 antagonizes tumor-derived TGF-β1 and restores the lymphokine-activated killing activity. J. Immunol. 172, 1508–1514 (2004).
Blaschitz, A., Hutter, H. & Dohr, G. HLA Class I protein expression in the human placenta. Early Pregnancy 5, 67–69 (2001).
Sasaki, N. & Idica, A. The HLA-matching effect in different cohorts of kidney transplant recipients: 10 years later. Clin. Transpl. 25, 261–282 (2010).
Sasazuki, T. et al. Effect of matching of class I HLA alleles on clinical outcome after transplantation of hematopoietic stem cells from an unrelated donor. Japan Marrow Donor Program. N. Engl. J. Med. 339, 1177–1185 (1998).
Lee, S. J. et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood 110, 4576–4583 (2007).
Zorn, E. et al. Minor histocompatibility antigen DBY elicits a coordinated B and T cell response after allogeneic stem cell transplantation. J. Exp. Med. 199, 1133–1142 (2004).
Perreault, C. et al. Minor histocompatibility antigens. Blood 76, 1269–1280 (1990).
Falkenburg, J. H., van de Corput, L., Marijt, E. W. & Willemze, R. Minor histocompatibility antigens in human stem cell transplantation. Exp. Hematol. 31, 743–751 (2003).
Warren, E. H. et al. Effect of MHC and non-MHC donor/recipient genetic disparity on the outcome of allogeneic HCT. Blood 120, 2796–2806 (2012).
Spierings, E. Minor histocompatibility antigens: past, present, and future. Tissue Antigens 84, 374–360 (2014).
Pye, R. J. et al. A second transmissible cancer in Tasmanian devils. Proc. Natl Acad. Sci. USA 113, 374–379 (2016).
Stammnitz, M. R. et al. The origins and vulnerabilities of two transmissible cancers in tasmanian devils. Cancer Cell 33, 607–619 (2018).
King, A. et al. Surface expression of HLA-C antigen by human extravillous trophoblast. Placenta 21, 376–387 (2000).
Turcotte, S. et al. Tumor MHC class I expression improves the prognostic value of T-cell density in resected colorectal liver metastases. Cancer Immunol. Res. 2, 530–537 (2014).
Iwayama, Y. et al. Prognostic value of HLA class I expression in patients with colorectal cancer. World J. Surg. Oncol. 13, 36 (2015).
Goeppert, B. et al. Major histocompatibility complex class I expression impacts on patient survival and type and density of immune cells in biliary tract cancer. Br. J. Cancer 113, 1343–1349 (2015).
Pinto, E. M. et al. Prognostic significance of major histocompatibility complex class II expression in pediatric adrenocortical tumors: a St. Jude and Children’s Oncology Group study. Clin. Cancer Res. 22, 6247–6255 (2016).
Durrant, L. G. et al. Quantitation of MHC antigen expression on colorectal tumours and its association with tumour progression. Br. J. Cancer 56, 425–432 (1987).
Oldford, S. A., Robb, J. D., Watson, P. H. & Drover, S. HLA-DRB alleles are differentially expressed by tumor cells in breast carcinoma. Int. J. Cancer 112, 399–406 (2004).
Park, I. A. et al. Expression of the MHC class II in triple-negative breast cancer is associated with tumor-infiltrating lymphocytes and interferon signaling. PLOS ONE 12, e0182786 (2017).
Lin, C. M. & Gill, R. G. Direct and indirect allograft recognition: pathways dictating graft rejection mechanisms. Curr. Opin. Organ Transplant. 21, 40–44 (2016).
Erlebacher, A., Vencato, D., Price, K. A., Zhang, D. & Glimcher, L. H. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J. Clin. Invest. 117, 1399–1411 (2007).
Collins, M. K., Tay, C. S. & Erlebacher, A. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J. Clin. Invest. 119, 2062–2073 (2009).
Volchek, M. et al. Lymphatics in the human endometrium disappear during decidualization. Hum. Reprod. 25, 2455–2464 (2010).
Kapsenberg, M. L. Dendritic-cell control of pathogen-driven T-cell polarization. Nat. Rev. Immunol. 3, 984–993 (2003).
Moldenhauer, L. M. et al. Cross-presentation of male seminal fluid antigens elicits T cell activation to initiate the female immune response to pregnancy. J. Immunol. 182, 8080–8093 (2009).
Rowe, J. H., Ertelt, J. M., Xin, L. & Way, S. S. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature 490, 102–106 (2012).
Samstein, R. M., Josefowicz, S. Z., Arvey, A., Treuting, P. M. & Rudensky, A. Y. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell 150, 29–38 (2012).
Aluvihare, V. R., Kallikourdis, M. & Betz, A. G. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 5, 266–271 (2004).
Shima, T. et al. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J. Reprod. Immunol. 85, 121–129 (2010).
Darrasse-Jeze, G., Klatzmann, D., Charlotte, F., Salomon, B. L. & Cohen, J. L. CD4+CD25+ regulatory/suppressor T cells prevent allogeneic fetus rejection in mice. Immunol. Lett. 102, 106–109 (2006).
Kauma, S. W., Huff, T. F., Hayes, N. & Nilkaeo, A. Placental Fas ligand expression is a mechanism for maternal immune tolerance to the fetus. J. Clin. Endocrinol. Metab. 84, 2188–2194 (1999).
Qiu, Q., Yang, M., Tsang, B. K. & Gruslin, A. Fas ligand expression by maternal decidual cells is negatively correlated with the abundance of leukocytes present at the maternal-fetal interface. J. Reprod. Immunol. 65, 121–132 (2005).
Hunt, J. S., Vassmer, D., Ferguson, T. A. & Miller, L. Fas ligand is positioned in mouse uterus and placenta to prevent trafficking of activated leukocytes between the mother and the conceptus. J. Immunol. 158, 4122–4128 (1997).
Guleria, I. et al. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J. Exp. Med. 202, 231–237 (2005).
Petroff, M. G. et al. B7 family molecules are favorably positioned at the human maternal-fetal interface. Biol. Reprod 68, 1496–1504 (2003).
Munn, D. H. et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281, 1191–1193 (1998).
Honig, A. et al. Indoleamine 2,3-dioxygenase (IDO) expression in invasive extravillous trophoblast supports role of the enzyme for materno-fetal tolerance. J. Reprod. Immunol. 61, 79–86 (2004).
Nancy, P. et al. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science 336, 1317–1321 (2012).
Martinez de la Torre, Y. et al. Protection against inflammation- and autoantibody-caused fetal loss by the chemokine decoy receptor D6. Proc. Natl Acad. Sci. USA 104, 2319–2324 (2007).
Madigan, J. et al. Chemokine scavenger D6 is expressed by trophoblasts and aids the survival of mouse embryos transferred into allogeneic recipients. J. Immunol. 184, 3202–3212 (2010).
Coulie, P. G., van den Eynde, B. J., van der Bruggen, P. & Boon, T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat. Rev. Cancer 14, 135–146 (2014).
Dunn, G. P., Koebel, C. M. & Schreiber, R. D. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 6, 836–848 (2006).
Gajewski, T. F., Schreiber, H. & Fu, Y. X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 14, 1014–1022 (2013).
Spranger, S. Mechanisms of tumor escape in the context of the T-cell-inflamed and the non-T-cell-inflamed tumor microenvironment. Int. Immunol. 28, 383–391 (2016).
Gabrilovich, D. I. & Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 (2009).
Gooden, M. J., de Bock, G. H., Leffers, N., Daemen, T. & Nijman, H. W. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br. J. Cancer 105, 93–103 (2011).
Balachandran, V. P. et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat. Med. 17, 1094–1100 (2011).
Medema, J. P. et al. Blockade of the granzyme B/perforin pathway through overexpression of the serine protease inhibitor PI-9/SPI-6 constitutes a mechanism for immune escape by tumors. Proc. Natl Acad. Sci. USA 98, 11515–11520 (2001).
Barclay, A. N. & van den Berg, T. K. The interaction between signal regulatory protein alpha (SIRPα) and CD47: structure, function, and therapeutic target. Annu. Rev. Immunol. 32, 25–50 (2014).
Matlung, H. L., Szilagyi, K., Barclay, N. A. & van den Berg, T. K. The CD47-SIRPα signaling axis as an innate immune checkpoint in cancer. Immunol. Rev. 276, 145–164 (2017).
Matozaki, T., Murata, Y., Okazawa, H. & Ohnishi, H. Functions and molecular mechanisms of the CD47-SIRPα signalling pathway. Trends Cell. Biol. 19, 72–80 (2009).
Nathan, C. & Muller, W. A. Putting the brakes on innate immunity: a regulatory role for CD200? Nat. Immunol. 2, 17–19 (2001).
Kretz-Rommel, A. et al. CD200 expression on tumor cells suppresses antitumor immunity: new approaches to cancer immunotherapy. J. Immunol. 178, 5595–5605 (2007).
Siva, A. et al. Immune modulation by melanoma and ovarian tumor cells through expression of the immunosuppressive molecule CD200. Cancer Immunol. Immunother. 57, 987–996 (2008).
Shields, J. D., Kourtis, I. C., Tomei, A. A., Roberts, J. M. & Swartz, M. A. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science 328, 749–752 (2010).
Bierie, B. & Moses, H. L. Tumour microenvironment: TGFβ: the molecular Jekyll and Hyde of cancer. Nat. Rev. Cancer 6, 506–520 (2006).
Flavell, R. A., Sanjabi, S., Wrzesinski, S. H. & Licona-Limon, P. The polarization of immune cells in the tumour environment by TGFβ. Nat. Rev. Immunol. 10, 554–567 (2010).
Belov, K. The role of the major histocompatibility complex in the spread of contagious cancers. Mamm. Genome 22, 83–90 (2011).
Decker, B. et al. Comparison against 186 canid whole-genome sequences reveals survival strategies of an ancient clonally transmissible canine tumor. Genome Res. 25, 1646–1655 (2015).
McSorley, H. J. & Maizels, R. M. Helminth infections and host immune regulation. Clin. Microbiol. Rev. 25, 585–608 (2012).
Gomez-Munoz, M. T. et al. Inhibition of bovine T lymphocyte responses by extracts of the stomach worm Ostertagia ostertagi. Vet. Parasitol. 120, 199–214 (2004).
Donnelly, S., O’Neill, S. M., Sekiya, M., Mulcahy, G. & Dalton, J. P. Thioredoxin peroxidase secreted by fasciola hepatica induces the alternative activation of macrophages. Infect. Immun. 73, 166–173 (2005).
Flynn, R. J. & Mulcahy, G. The roles of IL-10 and TGF-β in controlling IL-4 and IFN-γ production during experimental fasciola hepatica infection. Int. J. Parasitol. 38, 1673–1680 (2008).
Layland, L. E. et al. Pronounced phenotype in activated regulatory T cells during a chronic helminth infection. J. Immunol. 184, 713–724 (2010).
Grainger, J. R. et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J. Exp. Med. 207, 2331–2341 (2010).
Blankenhaus, B. et al. Strongyloides ratti infection induces expansion of Foxp3+ regulatory T cells that interfere with immune response and parasite clearance in BALB/c mice. J. Immunol. 186, 4295–4305 (2011).
Ludwig-Portugall, I. & Layland, L. E. TLRs, Treg, and B cells, an interplay of regulation during helminth infection. Front. Immunol. 3, 8 (2012).
Terrazas, C. A., Terrazas, L. I. & Gomez-Garcia, L. Modulation of dendritic cell responses by parasites: a common strategy to survive. J. Biomed. Biotechnol. 2010, 357106 (2010).
Smyth, D. J. et al. TGF-β mimic proteins form an extended gene family in the murine parasite heligmosomoides polygyrus. Int. J. Parasitol. 48, 379–385 (2018).
Johnston, C. J. C. et al. A structurally distinct TGF-β mimic from an intestinal helminth parasite potently induces regulatory T cells. Nat. Commun. 8, 1741 (2017).
Everts, B., Smits, H. H., Hokke, C. H. & Yazdanbakhsh, M. Helminths and dendritic cells: sensing and regulating via pattern recognition receptors, Th2 and Treg responses. Eur. J. Immunol. 40, 1525–1537 (2010).
Robinson, M. W. et al. A helminth cathelicidin-like protein suppresses antigen processing and presentation in macrophages via inhibition of lysosomal vATPase. FASEB J. 26, 4614–4627 (2012).
Maizels, R. M., Bundy, D. A., Selkirk, M. E., Smith, D. F. & Anderson, R. M. Immunological modulation and evasion by helminth parasites in human populations. Nature 365, 797–805 (1993).
Hewitson, J. P., Grainger, J. R. & Maizels, R. M. Helminth immunoregulation: the role of parasite secreted proteins in modulating host immunity. Mol. Biochem. Parasitol. 167, 1–11 (2009).
Liang, Q. et al. Linking a cell-division gene and a suicide gene to define and improve cell therapy safety. Nature 563, 701–704 (2018).
Hicklin, D. J. et al. β2-Microglobulin mutations, HLA class I antigen loss, and tumor progression in melanoma. J. Clin. Invest. 101, 2720–2729 (1998).
Hanna, S. & Etzioni, A. MHC class I and II deficiencies. J. Allergy Clin. Immunol. 134, 269–275 (2014).
Street, S. E. et al. Innate immune surveillance of spontaneous B cell lymphomas by natural killer cells and gammadelta T cells. J. Exp. Med. 199, 879–884 (2004).
Metzger, M. J. et al. Widespread transmission of independent cancer lineages within multiple bivalve species. Nature 534, 705–709 (2016).
Pearse, A. M. & Swift, K. Allograft theory: transmission of devil facial-tumour disease. Nature 439, 549 (2006).
Spriggs, M. K. et al. Beta 2-microglobulin-, CD8+ T-cell-deficient mice survive inoculation with high doses of vaccinia virus and exhibit altered IgG responses. Proc. Natl Acad. Sci. USA 89, 6070–6074 (1992).
Hou, S., Doherty, P. C., Zijlstra, M., Jaenisch, R. & Katz, J. M. Delayed clearance of Sendai virus in mice lacking class I MHC-restricted CD8+ T cells. J. Immunol. 149, 1319–1325 (1992).
Eichelberger, M., Allan, W., Zijlstra, M., Jaenisch, R. & Doherty, P. C. Clearance of influenza virus respiratory infection in mice lacking class I major histocompatibility complex-restricted CD8+ T cells. J. Exp. Med. 174, 875–880 (1991).
Neal, Z. C. & Splitter, G. A. Protection against lethal encephalomyocarditis virus infection in the absence of serum-neutralizing antibodies. J. Virol. 72, 8052–8060 (1998).
Bodmer, H., Obert, G., Chan, S., Benoist, C. & Mathis, D. Environmental modulation of the autonomy of cytotoxic T lymphocytes. Eur. J. Immunol. 23, 1649–1654 (1993).
Laufer, T. M., von Herrath, M. G., Grusby, M. J., Oldstone, M. B. & Glimcher, L. H. Autoimmune diabetes can be induced in transgenic major histocompatibility complex class II-deficient mice. J. Exp. Med. 178, 589–596 (1993).
Gargett, T. & Brown, M. P. The inducible caspase-9 suicide gene system as a “safety switch” to limit on-target, off-tumor toxicities of chimeric antigen receptor T cells. Front. Pharmacol. 5, 235 (2014).
Atala, A., Lanza, R., Mikos, A. G. & Nerem, R. Principles of Regenerative Medicine 3rd Edition (Academic Press, London/San Diego, 2019).
Lanza, R., Langer, R. & Vacanti, J. Principles of Tissue Engineering 4th Edition (Academic Press, London/San Diego, 2014).
Acknowledgements
The authors thank J. Harding for his critical and helpful input.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
R.L., D.W.R. and A.N. are employees and/or founders of the Astellas Institute for Regenerative Medicine, Universal Cells Inc. and panCELLa Inc., respectively, companies in the area of regenerative medicine, including the generation of cell therapies and universal cells.
Additional information
Peer review informationNature Reviews Immunology thanks Sonia Schrepfer, Xang Xu and Rainer Blasczyk for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Intrabody
-
An antibody that works within cells to bind to a specific intracellular protein.
- Cross-presentation
-
The uptake and presentation of antigens by cells that do not express those antigens themselves.
- ‘Direct recognition’ pathway
-
Host T cells can rapidly recognize a donor antigen that is being presented directly by donor antigen-presenting cells that are present during transplant. As opposed to indirect recognition, where host T cells recognize an antigen that is being presented by host antigen-presenting cells, which usually takes more time because the host has to acquire and process donor antigen.
- iCasp9
-
The dimerizing small drug-inducible Caspase 9 system kills cells by initiating apoptosis.
- Herpes simplex virus thymidine kinase
-
A commonly used suicide gene. Cells expressing herpes simplex virus thymidine kinase die in the presence of gancyclovir.
Rights and permissions
About this article
Cite this article
Lanza, R., Russell, D.W. & Nagy, A. Engineering universal cells that evade immune detection. Nat Rev Immunol 19, 723–733 (2019). https://doi.org/10.1038/s41577-019-0200-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-019-0200-1