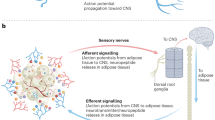
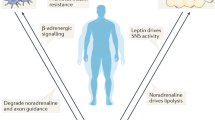
Abstract
Adipose tissue comprises adipocytes and many other cell types that engage in dynamic crosstalk in a highly innervated and vascularized tissue matrix. Although adipose tissue has been studied for decades, it has been appreciated only in the past 5 years that extensive arborization of nerve fibres has a dominant role in regulating the function of adipose tissue. This Review summarizes the latest literature, which suggests that adipocytes signal to local sensory nerve fibres in response to perturbations in lipolysis and lipogenesis. Such adipocyte signalling to the central nervous system causes sympathetic output to distant adipose depots and potentially other metabolic tissues to regulate systemic glucose homeostasis. Paracrine factors identified in the past few years that mediate such adipocyte–neuron crosstalk are also reviewed. Similarly, immune cells and endothelial cells within adipose tissue communicate with local nerve fibres to modulate neurotransmitter tone, blood flow, adipocyte differentiation and energy expenditure, including adipose browning to produce heat. This understudied field of neurometabolism related to adipose tissue biology has great potential to reveal new mechanistic insights and potential therapeutic strategies for obesity and type 2 diabetes mellitus.
Key points
-
Adipocytes modulate whole-body metabolism through secretion of endocrine and paracrine factors that modulate local immune cell cytokine secretion, endothelium blood flow and neuronal signalling to the brain.
-
Adipocytes, the endothelium and immune cells within adipose tissues secrete factors such as leptin, vascular endothelial growth factor (VEGF) and tumour necrosis factor (TNF) that regulate local sensory nerve fibre functions.
-
Adipocyte lipid metabolism communicates with local sensory nerve fibres, sending signals to the central nervous system; conversely, sensory nerve fibres secrete factors such as calcitonin-related gene peptide (CGRP) and substance P that might regulate the metabolism of adipocytes and other adipose-resident cells.
-
Increased lipolysis in white adipose tissue in response to sympathetic activation can cause sensory nerve fibres to regulate the metabolic activity of distant brown adipose tissue depots.
-
Extensive and dynamic signalling networks among the diverse cell types in adipose tissue integrate and mediate communication through bioactive lipids to local sensory nerve fibres and neurotrophic factors to sympathetic nerve fibres.
-
Identifying factors within adipose tissues that regulate the function of sensory and sympathetic nerve fibres might reveal therapeutic strategies for obesity and type 2 diabetes mellitus.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Shaw, H. B. A. Contribution to the study of the morphology of adipose tissue. J. Anat. Physiol. 36, 1–13 (1901).
Kershaw, E. E. & Flier, J. S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 89, 2548–2556 (2004).
Ouchi, N., Parker, J. L., Lugus, J. J. & Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 11, 85–97 (2011).
Villarroya, F., Cereijo, R., Villarroya, J. & Giralt, M. Brown adipose tissue as a secretory organ. Nat. Rev. Endocrinol. 13, 26–35 (2017).
Stern, J. H., Rutkowski, J. M. & Scherer, P. E. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 23, 770–784 (2016).
Shin, J. et al. SDF-1 is an autocrine insulin-desensitizing factor in adipocytes. Diabetes 67, 1068–1078 (2018).
Villarroya, F., Gavalda-Navarro, A., Peyrou, M., Villarroya, J. & Giralt, M. The lives and times of brown adipokines. Trends Endocrinol. Metab. 28, 855–867 (2017).
Cannon, B. & Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 (2004).
Rosen, E. D. & Spiegelman, B. M. What we talk about when we talk about fat. Cell 156, 20–44 (2014).
Guilherme, A., Virbasius, J. V., Puri, V. & Czech, M. P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 9, 367–377 (2008).
Nye, C., Kim, J., Kalhan, S. C. & Hanson, R. W. Reassessing triglyceride synthesis in adipose tissue. Trends Endocrinol. Metab. 19, 356–361 (2008).
Teusink, B. et al. Contribution of fatty acids released from lipolysis of plasma triglycerides to total plasma fatty acid flux and tissue-specific fatty acid uptake. Diabetes 52, 614–620 (2003).
Kersten, S. Physiological regulation of lipoprotein lipase. Biochim. Biophys. Acta 1841, 919–933 (2014).
Czech, M. P., Tencerova, M., Pedersen, D. J. & Aouadi, M. Insulin signalling mechanisms for triacylglycerol storage. Diabetologia 56, 949–964 (2013).
Unger, R. H., Clark, G. O., Scherer, P. E. & Orci, L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim. Biophys. Acta 1801, 209–214 (2010).
Perry, R. J. et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 160, 745–758 (2015).
Titchenell, P. M. et al. Direct hepatocyte insulin signaling is required for lipogenesis but is dispensable for the suppression of glucose production. Cell Metab. 23, 1154–1166 (2016).
Clerk, L. H., Rattigan, S. & Clark, M. G. Lipid infusion impairs physiologic insulin-mediated capillary recruitment and muscle glucose uptake in vivo. Diabetes 51, 1138–1145 (2002).
Dresner, A. et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J. Clin. Invest. 103, 253–259 (1999).
Petersen, K. F. & Shulman, G. I. Cellular mechanism of insulin resistance in skeletal muscle. J. R. Soc. Med. 95 (Suppl. 42), 8–13 (2002).
DeFronzo, R. A., Bonadonna, R. C. & Ferrannini, E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care 15, 318–368 (1992).
Walther, T. C., Chung, J. & Farese, R. V. Jr. Lipid droplet biogenesis. Annu. Rev. Cell Dev. Biol. 33, 491–510 (2017).
Cinti, S. et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 46, 2347–2355 (2005).
Kanda, H. et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Invest. 116, 1494–1505 (2006).
Amano, S. U. et al. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 19, 162–171 (2014).
Weisberg, S. P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 (2003).
Xu, H. et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830 (2003).
Gustafson, B. & Smith, U. Cytokines promote Wnt signaling and inflammation and impair the normal differentiation and lipid accumulation in 3T3-L1 preadipocytes. J. Biol. Chem. 281, 9507–9516 (2006).
Grant, R. W. & Stephens, J. M. Fat in flames: influence of cytokines and pattern recognition receptors on adipocyte lipolysis. Am. J. Physiol. Endocrinol. Metab. 309, E205–E213 (2015).
Aouadi, M. et al. Gene silencing in adipose tissue macrophages regulates whole-body metabolism in obese mice. Proc. Natl Acad. Sci. USA 110, 8278–8283 (2013).
Sung, H. K. et al. Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell Metab. 17, 61–72 (2013).
Halaas, J. L. et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269, 543–546 (1995).
Bartness, T. J., Kay Song, C., Shi, H., Bowers, R. R. & Foster, M. T. Brain-adipose tissue cross talk. Proc. Nutr. Soc. 64, 53–64 (2005).
Bartness, T. & Kay Song, C. Innervation of brown adipose tissue and its role in thermogenesis. Can. J. Diabetes 29, 420–428 (2005).
Bartness, T. J. & Ryu, V. Neural control of white, beige and brown adipocytes. Int. J. Obes. Suppl. 5, S35–S39 (2015).
Caron, A., Lee, S., Elmquist, J. K. & Gautron, L. Leptin and brain-adipose crosstalks. Nat. Rev. Neurosci. 19, 153–165 (2018).
Friedman, J. 20 years of leptin: leptin at 20: an overview. J. Endocrinol. 223, T1–T8 (2014).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994).
Ryu, V., Garretson, J. T., Liu, Y., Vaughan, C. H. & Bartness, T. J. Brown adipose tissue has sympathetic-sensory feedback circuits. J. Neurosci. 35, 2181–2190 (2015).
Garretson, J. T. et al. Lipolysis sensation by white fat afferent nerves triggers brown fat thermogenesis. Mol. Metab. 5, 626–634 (2016).
Harris, R. B. S. Denervation as a tool for testing sympathetic control of white adipose tissue. Physiol. Behav. 190, 3–10 (2018).
Fishman, R. B. & Dark, J. Sensory innervation of white adipose tissue. Am. J. Physiol. 253, R942–R944 (1987).
Shi, H., Song, C. K., Giordano, A., Cinti, S. & Bartness, T. J. Sensory or sympathetic white adipose tissue denervation differentially affects depot growth and cellularity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R1028–R1037 (2005).
De Matteis, R., Ricquier, D. & Cinti, S. TH-, NPY-, SP-, and CGRP-immunoreactive nerves in interscapular brown adipose tissue of adult rats acclimated at different temperatures: an immunohistochemical study. J. Neurocytol. 27, 877–886 (1998).
Song, C. K., Schwartz, G. J. & Bartness, T. J. Anterograde transneuronal viral tract tracing reveals central sensory circuits from white adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R501–R511 (2009).
Vaughan, C. H. & Bartness, T. J. Anterograde transneuronal viral tract tracing reveals central sensory circuits from brown fat and sensory denervation alters its thermogenic responses. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302, R1049–R1058 (2012).
Ryu, V. & Bartness, T. J. Short and long sympathetic-sensory feedback loops in white fat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 306, R886–R900 (2014).
Nguyen, N. L. T., Xue, B. & Bartness, T. J. Sensory denervation of inguinal white fat modifies sympathetic outflow to white and brown fat in Siberian hamsters. Physiol. Behav. 190, 28–33 (2018).
Guilherme, A. et al. Neuronal modulation of brown adipose activity through perturbation of white adipocyte lipogenesis. Mol. Metab. 16, 116–125 (2018).
Pereira, M. M. et al. A brain-sparing diphtheria toxin for chemical genetic ablation of peripheral cell lineages. Nat. Commun. 8, 14967 (2017).
Niijima, A. Afferent signals from leptin sensors in the white adipose tissue of the epididymis, and their reflex effect in the rat. J. Auton. Nerv. Syst. 73, 19–25 (1998).
Niijima, A. Reflex effects from leptin sensors in the white adipose tissue of the epididymis to the efferent activity of the sympathetic and vagus nerve in the rat. Neurosci. Lett. 262, 125–128 (1999).
Murphy, K. T. et al. Leptin-sensitive sensory nerves innervate white fat. Am. J. Physiol. Endocrinol. Metab. 304, E1338–E1347 (2013).
de Lartigue, G., Ronveaux, C. C. & Raybould, H. E. Deletion of leptin signaling in vagal afferent neurons results in hyperphagia and obesity. Mol. Metab. 3, 595–607 (2014).
Zeng, W. et al. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell 163, 84–94 (2015).
Shiuchi, T. et al. Induction of glucose uptake in skeletal muscle by central leptin is mediated by muscle beta2-adrenergic receptor but not by AMPK. Sci. Rep. 7, 15141 (2017).
Waxman, S. G. From Neuroscience to Neurology: Neuroscience, Molecular Medicine, and the Therapeutic Transformation of Neurology (Elsevier Academic Press, 2005).
Korsching, S. The neurotrophic factor concept: a reexamination. J. Neurosci. 13, 2739–2748 (1993).
Christian, M. Transcriptional fingerprinting of “browning” white fat identifies NRG4 as a novel adipokine. Adipocyte 4, 50–54 (2015).
Rosell, M. et al. Brown and white adipose tissues: intrinsic differences in gene expression and response to cold exposure in mice. Am. J. Physiol. Endocrinol. Metab. 306, E945–E964 (2014).
Pellegrinelli, V. et al. Adipocyte-secreted BMP8b mediates adrenergic-induced remodeling of the neuro-vascular network in adipose tissue. Nat. Commun. 9, 4974 (2018).
Nakagomi, A. et al. Role of the central nervous system and adipose tissue BDNF/TrkB axes in metabolic regulation. NPJ Aging Mech. Dis. 1, 15009 (2015).
Cao, Y., Wang, H. & Zeng, W. Whole-tissue 3D imaging reveals intra-adipose sympathetic plasticity regulated by NGF-TrkA signal in cold-induced beiging. Protein Cell 9, 527–539 (2018).
Wang, G. X. et al. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat. Med. 20, 1436–1443 (2014).
Min, S. Y. et al. Human ‘brite/beige’ adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nat. Med. 22, 312–318 (2016).
Pearson, R. J. Jr & Carroll, S. L. ErbB transmembrane tyrosine kinase receptors are expressed by sensory and motor neurons projecting into sciatic nerve. J. Histochem. Cytochem. 52, 1299–1311 (2004).
Vetter, I., Pujic, Z. & Goodhill, G. J. The response of dorsal root ganglion axons to nerve growth factor gradients depends on spinal level. J. Neurotrauma 27, 1379–1386 (2010).
Moy, J. K., Khoutorsky, A., Asiedu, M. N., Dussor, G. & Price, T. J. eIF4E phosphorylation influences Bdnf mRNA translation in mouse dorsal root ganglion neurons. Front. Cell. Neurosci. 12, 29 (2018).
Cannon, B. et al. ‘Neuropeptide tyrosine’ (NPY) is co-stored with noradrenaline in vascular but not in parenchymal sympathetic nerves of brown adipose tissue. Exp. Cell Res. 164, 546–550 (1986).
Slavin, B. G. & Ballard, K. W. Morphological studies on the adrenergic innervation of white adipose tissue. Anat. Rec. 191, 377–389 (1978).
Bartness, T. J. & Bamshad, M. Innervation of mammalian white adipose tissue: implications for the regulation of total body fat. Am. J. Physiol. 275, R1399–R1411 (1998).
Carmeliet, P. & Tessier-Lavigne, M. Common mechanisms of nerve and blood vessel wiring. Nature 436, 193–200 (2005).
Shvartsman, D. et al. Sustained delivery of VEGF maintains innervation and promotes reperfusion in ischemic skeletal muscles via NGF/GDNF signaling. Mol. Ther. 22, 1243–1253 (2014).
Mackenzie, F. & Ruhrberg, C. Diverse roles for VEGF-A in the nervous system. Development 139, 1371–1380 (2012).
Guaiquil, V. H. et al. VEGF-B selectively regenerates injured peripheral neurons and restores sensory and trophic functions. Proc. Natl Acad. Sci. USA 111, 17272–17277 (2014).
Park, J. et al. VEGF-A-expressing adipose tissue shows rapid beiging and enhanced survival after transplantation and confers IL-4-independent metabolic improvements. Diabetes 66, 1479–1490 (2017).
Sun, K. et al. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc. Natl Acad. Sci. USA 109, 5874–5879 (2012).
Dhondt, J. et al. Neuronal FLT1 receptor and its selective ligand VEGF-B protect against retrograde degeneration of sensory neurons. FASEB J. 25, 1461–1473 (2011).
Pedersen, D. J. et al. A major role of insulin in promoting obesity-associated adipose tissue inflammation. Mol. Metab. 4, 507–518 (2015).
Hardy, O. T. et al. Body mass index-independent inflammation in omental adipose tissue associated with insulin resistance in morbid obesity. Surg. Obes. Relat. Dis. 7, 60–67 (2011).
Zanos, T. P. et al. Identification of cytokine-specific sensory neural signals by decoding murine vagus nerve activity. Proc. Natl Acad. Sci. USA 115, E4843–E4852 (2018).
Osamura, N. et al. Induction of interleukin-6 in dorsal root ganglion neurons after gradual elongation of rat sciatic nerve. Exp. Neurol. 195, 61–70 (2005).
Miller, R. J., Jung, H., Bhangoo, S. K. & White, F. A. Cytokine and chemokine regulation of sensory neuron function. Handb. Exp. Pharmacol. 194, 417–449 (2009).
Nunez Ruiz, A. et al. Diminished levels of regulatory T cell subsets (CD8+Foxp3, CD4+Foxp3 and CD4+CD39+Foxp3) but increased Foxp3 expression in adipose tissue from overweight subjects. Nutrition 32, 943–954 (2016).
Cortez-Espinosa, N. et al. CD39 expression on Treg and Th17 cells is associated with metabolic factors in patients with type 2 diabetes. Hum. Immunol. 76, 622–630 (2015).
Herbert, M. K. & Holzer, P. Neurogenic inflammation. I. Basic mechanisms, physiology and pharmacology [German]. Anasthesiol. Intensivmed. Notfallmed. Schmerzther. 37, 314–325 (2002).
Russell, F. A., King, R., Smillie, S. J., Kodji, X. & Brain, S. D. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol. Rev. 94, 1099–1142 (2014).
Brain, S. D. & Grant, A. D. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol. Rev. 84, 903–934 (2004).
Sung, C. P. et al. CGRP stimulates the adhesion of leukocytes to vascular endothelial cells. Peptides 13, 429–434 (1992).
Liu, T. et al. Endogenous calcitonin gene-related peptide regulates lipid metabolism and energy homeostasis in male mice. Endocrinology 158, 1194–1206 (2017).
Walker, C. S. et al. Mice lacking the neuropeptide alpha-calcitonin gene-related peptide are protected against diet-induced obesity. Endocrinology 151, 4257–4269 (2010).
Aveseh, M., Koushkie-Jahromi, M., Nemati, J. & Esmaeili-Mahani, S. Serum calcitonin gene-related peptide facilitates adipose tissue lipolysis during exercise via PIPLC/IP3 pathways. Endocrine 61, 462–472 (2018).
Johnson, M. B., Young, A. D. & Marriott, I. The therapeutic potential of targeting substance P/NK-1R interactions in inflammatory CNS disorders. Front. Cell. Neurosci. 10, 296 (2016).
Garcia-Recio, S. & Gascon, P. Biological and pharmacological aspects of the NK1-receptor. Biomed. Res. Int. 2015, 495704 (2015).
O’Connor, T. M. et al. The role of substance P in inflammatory disease. J. Cell. Physiol. 201, 167–180 (2004).
Reilly, S. M. & Saltiel, A. R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 13, 633–643 (2017).
Ellis, A. & Bennett, D. L. Neuroinflammation and the generation of neuropathic pain. Br. J. Anaesth. 111, 26–37 (2013).
Yadav, R. L. et al. Somatic neural alterations in non-diabetic obesity: a cross-sectional study. BMC Obes. 3, 50 (2016).
Richardson, J. D. & Vasko, M. R. Cellular mechanisms of neurogenic inflammation. J. Pharmacol. Exp. Ther. 302, 839–845 (2002).
Xanthos, D. N. & Sandkuhler, J. Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat. Rev. Neurosci. 15, 43–53 (2014).
Schaper, N. C., Huijberts, M. & Pickwell, K. Neurovascular control and neurogenic inflammation in diabetes. Diabetes Metab. Res. Rev. 24, S40–S44 (2008).
Farkas, G. J. & Gater, D. R. Neurogenic obesity and systemic inflammation following spinal cord injury: a review. J. Spinal Cord Med. 41, 378–387 (2017).
Morrison, S. F., Madden, C. J. & Tupone, D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab. 19, 741–756 (2014).
Morrison, S. F. Central neural control of thermoregulation and brown adipose tissue. Auton. Neurosci. 196, 14–24 (2016).
Williams, K. W. & Elmquist, J. K. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat. Neurosci. 15, 1350–1355 (2012).
Konner, A. C., Klockener, T. & Bruning, J. C. Control of energy homeostasis by insulin and leptin: targeting the arcuate nucleus and beyond. Physiol. Behav. 97, 632–638 (2009).
Timper, K. & Bruning, J. C. Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis. Model. Mech. 10, 679–689 (2017).
Hollenberg, A. N. The role of the thyrotropin-releasing hormone (TRH) neuron as a metabolic sensor. Thyroid 18, 131–139 (2008).
Ryu, V., Watts, A. G., Xue, B. & Bartness, T. J. Bidirectional crosstalk between the sensory and sympathetic motor systems innervating brown and white adipose tissue in male Siberian hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 312, R324–R337 (2017).
Nguyen, N. L., Randall, J., Banfield, B. W. & Bartness, T. J. Central sympathetic innervations to visceral and subcutaneous white adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 306, R375–R386 (2014).
Bamshad, M., Aoki, V. T., Adkison, M. G., Warren, W. S. & Bartness, T. J. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am. J. Physiol. 275, R291–R299 (1998).
Morton, G. J. & Schwartz, M. W. Leptin and the central nervous system control of glucose metabolism. Physiol. Rev. 91, 389–411 (2011).
Contreras, C., Nogueiras, R., Dieguez, C., Rahmouni, K. & Lopez, M. Traveling from the hypothalamus to the adipose tissue: the thermogenic pathway. Redox Biol. 12, 854–863 (2017).
Myers, M. G. Jr & Olson, D. P. Central nervous system control of metabolism. Nature 491, 357–363 (2012).
Mahu, I. & Domingos, A. I. The sympathetic neuro-adipose connection and the control of body weight. Exp. Cell Res. 360, 27–30 (2017).
Dodd, G. T. & Tiganis, T. Insulin action in the brain: Roles in energy and glucose homeostasis. J. Neuroendocrinol. 29, e12513 (2017).
Roh, E., Song, D. K. & Kim, M. S. Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism. Exp. Mol. Med. 48, e216 (2016).
Benoit, S. C. et al. The catabolic action of insulin in the brain is mediated by melanocortins. J. Neurosci. 22, 9048–9052 (2002).
Choudhury, A. I. et al. The role of insulin receptor substrate 2 in hypothalamic and beta cell function. J. Clin. Invest. 115, 940–950 (2005).
Dodd, G. T. et al. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell 160, 88–104 (2015).
Dodd, G. T. et al. A hypothalamic phosphatase switch coordinates energy expenditure with feeding. Cell Metab. 26, 375–393 (2017).
Shin, A. C. et al. Insulin receptor signaling in POMC, but not AgRP, neurons controls adipose tissue insulin action. Diabetes 66, 1560–1571 (2017).
Harris, R. B., Kelso, E. W., Flatt, W. P., Bartness, T. J. & Grill, H. J. Energy expenditure and body composition of chronically maintained decerebrate rats in the fed and fasted condition. Endocrinology 147, 1365–1376 (2006).
Thaler, J. P. et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest. 122, 153–162 (2012).
Kleinridders, A. et al. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab. 10, 249–259 (2009).
Thaler, J. P. & Schwartz, M. W. Minireview: inflammation and obesity pathogenesis: the hypothalamus heats up. Endocrinology 151, 4109–4115 (2010).
Gao, Y. et al. Hormones and diet, but not body weight, control hypothalamic microglial activity. Glia 62, 17–25 (2014).
Garcia-Caceres, C., Yi, C. X. & Tschop, M. H. Hypothalamic astrocytes in obesity. Endocrinol. Metab. Clin. North Am. 42, 57–66 (2013).
Baufeld, C., Osterloh, A., Prokop, S., Miller, K. R. & Heppner, F. L. High-fat diet-induced brain region-specific phenotypic spectrum of CNS resident microglia. Acta Neuropathol. 132, 361–375 (2016).
Schur, E. A. et al. Radiologic evidence that hypothalamic gliosis is associated with obesity and insulin resistance in humans. Obesity 23, 2142–2148 (2015).
Valdearcos, M. et al. Microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metab. 26, 185–197 (2017).
Giordano, A. et al. White adipose tissue lacks significant vagal innervation and immunohistochemical evidence of parasympathetic innervation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R1243–R1255 (2006).
Bartness, T. J., Liu, Y., Shrestha, Y. B. & Ryu, V. Neural innervation of white adipose tissue and the control of lipolysis. Front. Neuroendocrinol. 35, 473–493 (2014).
Scherer, T. et al. Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell Metab. 13, 183–194 (2011).
Vitali, A. et al. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J. Lipid Res. 53, 619–629 (2012).
Jiang, H., Ding, X., Cao, Y., Wang, H. & Zeng, W. Dense intra-adipose sympathetic arborizations are essential for cold-induced beiging of mouse white adipose tissue. Cell Metab. 26, 686–692 (2017).
Chi, J. et al. Three-dimensional adipose tissue imaging reveals regional variation in beige fat biogenesis and PRDM16-dependent sympathetic neurite density. Cell Metab. 27, 226–236 (2018).
Schulz, T. J. et al. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature 495, 379–383 (2013).
Zhu, Y. et al. Connexin 43 mediates white adipose tissue beiging by facilitating the propagation of sympathetic neuronal signals. Cell Metab. 24, 420–433 (2016).
Burnstock, G. & Sneddon, P. Evidence for ATP and noradrenaline as cotransmitters in sympathetic nerves. Clin. Sci. 68, 89s–92s (1985).
Pablo Huidobro-Toro, J. & Veronica Donoso, M. Sympathetic co-transmission: the coordinated action of ATP and noradrenaline and their modulation by neuropeptide Y in human vascular neuroeffector junctions. Eur. J. Pharmacol. 500, 27–35 (2004).
Ralevic, V. & Dunn, W. R. Purinergic transmission in blood vessels. Auton. Neurosci. 191, 48–66 (2015).
Razzoli, M. et al. Stress-induced activation of brown adipose tissue prevents obesity in conditions of low adaptive thermogenesis. Mol. Metab. 5, 19–33 (2016).
Xie, T. R., Liu, C. F. & Kang, J. S. Sympathetic transmitters control thermogenic efficacy of brown adipocytes by modulating mitochondrial complex V. Signal Transduct. Target. Ther. 2, 17060 (2017).
Pirzgalska, R. M. et al. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat. Med. 23, 1309–1318 (2017).
Tang, L. et al. Sympathetic nerve activity maintains an anti-inflammatory state in adipose tissue in male mice by inhibiting TNF-alpha gene expression in macrophages. Endocrinology 156, 3680–3694 (2015).
Camell, C. D. et al. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature 550, 119–123 (2017).
Collins, S. Beta-adrenoceptor signaling networks in adipocytes for recruiting stored fat and energy expenditure. Front. Endocrinol. 2, 102 (2011).
Zechner, R., Madeo, F. & Kratky, D. Cytosolic lipolysis and lipophagy: two sides of the same coin. Nat. Rev. Mol. Cell Biol. 18, 671–684 (2017).
Granneman, J. G., Li, P., Zhu, Z. & Lu, Y. Metabolic and cellular plasticity in white adipose tissue I: effects of beta3-adrenergic receptor activation. Am. J. Physiol. Endocrinol. Metab. 289, E608–E616 (2005).
Ramseyer, V. D. & Granneman, J. G. Adrenergic regulation of cellular plasticity in brown, beige/brite and white adipose tissues. Adipocyte 5, 119–129 (2016).
Bachman, E. S. et al. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science 297, 843–845 (2002).
Douris, N. et al. Beta-adrenergic receptors are critical for weight loss but not for other metabolic adaptations to the consumption of a ketogenic diet in male mice. Mol. Metab. 6, 854–862 (2017).
Nisoli, E., Tonello, C., Briscini, L. & Carruba, M. O. Inducible nitric oxide synthase in rat brown adipocytes: implications for blood flow to brown adipose tissue. Endocrinology 138, 676–682 (1997).
Petrovic, V. et al. NO modulates the molecular basis of rat interscapular brown adipose tissue thermogenesis. Comp. Biochem. Physiol. C 152, 147–159 (2010).
Takahashi, H. et al. Beta-3 adrenergic agonist, BRL-26830A, and alpha/beta blocker, arotinolol, markedly increase regional blood flow in the brown adipose tissue in anesthetized rats. Jpn Circ. J. 56, 936–942 (1992).
Giordano, A. et al. Evidence for a functional nitric oxide synthase system in brown adipocyte nucleus. FEBS Lett. 514, 135–140 (2002).
Sipe, L. M. et al. Differential sympathetic outflow to adipose depots is required for visceral fat loss in response to calorie restriction. Nutr. Diabetes 7, e260 (2017).
Zhang, W., Cline, M. A. & Gilbert, E. R. Hypothalamus-adipose tissue crosstalk: neuropeptide Y and the regulation of energy metabolism. Nutr. Metab. 11, 27 (2014).
Turtzo, L. C., Marx, R. & Lane, M. D. Cross-talk between sympathetic neurons and adipocytes in coculture. Proc. Natl Acad. Sci. USA 98, 12385–12390 (2001).
Yang, K., Guan, H., Arany, E., Hill, D. J. & Cao, X. Neuropeptide Y is produced in visceral adipose tissue and promotes proliferation of adipocyte precursor cells via the Y1 receptor. FASEB J. 22, 2452–2464 (2008).
Kuo, L. E. et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat. Med. 13, 803–811 (2007).
Burnstock, G. Purinergic cotransmission. Exp. Physiol. 94, 20–24 (2009).
Burnstock, G. & Gentile, D. The involvement of purinergic signalling in obesity. Purinergic Signal. 14, 97–108 (2018).
Tozzi, M. & Novak, I. Purinergic receptors in adipose tissue as potential targets in metabolic disorders. Front. Pharmacol. 8, 878 (2017).
Ussar, S. et al. ASC-1, PAT2, and P2RX5 are cell surface markers for white, beige, and brown adipocytes. Sci. Transl Med. 6, 247ra103 (2014).
Stefanidis, A. et al. Insights into the neurochemical signature of the innervation of beige fat. Mol. Metab. 11, 47–58 (2018).
Bernhard, F. et al. Functional relevance of genes implicated by obesity genome-wide association study signals for human adipocyte biology. Diabetologia 56, 311–322 (2013).
Guilherme, A. et al. Adipocyte lipid synthesis coupled to neuronal control of thermogenic programming. Mol. Metab. 6, 781–796 (2017).
Migrenne, S. et al. Fatty acid signaling in the hypothalamus and the neural control of insulin secretion. Diabetes 55, 5 (2006).
Bazinet, R. P. & Laye, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 15, 771–785 (2014).
Matias, I. et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J. Clin. Endocrinol. Metab. 91, 3171–3180 (2006).
Gonthier, M. P. et al. Identification of endocannabinoids and related compounds in human fat cells. Obesity 15, 837–845 (2007).
Matias, I. et al. Role and regulation of acylethanolamides in energy balance: focus on adipocytes and beta-cells. Br. J. Pharmacol. 152, 676–690 (2007).
Ruiz de Azua, I. et al. Adipocyte cannabinoid receptor CB1 regulates energy homeostasis and alternatively activated macrophages. J. Clin. Invest. 127, 4148–4162 (2017).
Cote, M. et al. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int. J. Obes. 31, 692–699 (2007).
Di Marzo, V. et al. Changes in plasma endocannabinoid levels in viscerally obese men following a 1 year lifestyle modification programme and waist circumference reduction: associations with changes in metabolic risk factors. Diabetologia 52, 213–217 (2009).
Wagner, I. V., Perwitz, N., Drenckhan, M., Lehnert, H. & Klein, J. Cannabinoid type 1 receptor mediates depot-specific effects on differentiation, inflammation and oxidative metabolism in inguinal and epididymal white adipocytes. Nutr. Diabetes 1, e16 (2011).
Quarta, C. et al. CB(1) signaling in forebrain and sympathetic neurons is a key determinant of endocannabinoid actions on energy balance. Cell Metab. 11, 273–285 (2010).
Bajzer, M. et al. Cannabinoid receptor 1 (CB1) antagonism enhances glucose utilisation and activates brown adipose tissue in diet-induced obese mice. Diabetologia 54, 3121–3131 (2011).
Pakdeechote, P., Dunn, W. R. & Ralevic, V. Cannabinoids inhibit noradrenergic and purinergic sympathetic cotransmission in the rat isolated mesenteric arterial bed. Br. J. Pharmacol. 152, 725–733 (2007).
O’Keefe, L., Simcocks, A. C., Hryciw, D. H., Mathai, M. L. & McAinch, A. J. The cannabinoid receptor 1 and its role in influencing peripheral metabolism. Diabetes Obes. Metab. 16, 294–304 (2014).
Pongratz, G. & Straub, R. H. The sympathetic nervous response in inflammation. Arthritis Res. Ther. 16, 504 (2014).
Xiong, Y. et al. Long-acting MIC-1/GDF15 molecules to treat obesity: evidence from mice to monkeys. Sci. Transl Med. 9, eaan8732 (2017).
Rines, A. K., Verdeguer, F. & Puigserver, P. Adenosine activates thermogenic adipocytes. Cell Res. 25, 155–156 (2015).
Bootcov, M. R. et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc. Natl Acad. Sci. USA 94, 11514–11519 (1997).
Tsai, V. W., Lin, S., Brown, D. A., Salis, A. & Breit, S. N. Anorexia-cachexia and obesity treatment may be two sides of the same coin: role of the TGF-b superfamily cytokine MIC-1/GDF15. Int. J. Obes. 40, 193–197 (2016).
Tsai, V. W. et al. Treatment with the TGF-b superfamily cytokine MIC-1/GDF15 reduces the adiposity and corrects the metabolic dysfunction of mice with diet-induced obesity. Int. J. Obes. 42, 561–571 (2018).
O’Rahilly, S. GDF15 — from biomarker to allostatic hormone. Cell Metab. 26, 807–808 (2017).
Strelau, J., Schober, A., Sullivan, A., Schilling, L. & Unsicker, K. Growth/differentiation factor-15 (GDF-15), a novel member of the TGF-beta superfamily, promotes survival of lesioned mesencephalic dopaminergic neurons in vitro and in vivo and is induced in neurons following cortical lesioning. J. Neural Transm. Suppl. 65, 197–203 (2003).
Gnad, T. et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 516, 395–399 (2014).
Dobson, J. G. Jr Mechanism of adenosine inhibition of catecholamine-induced responses in heart. Circ. Res. 52, 151–160 (1983).
Rongen, G. A. et al. Presynaptic inhibition of norepinephrine release from sympathetic nerve endings by endogenous adenosine. Hypertension 27, 933–938 (1996).
Thorp, A. A. & Schlaich, M. P. Relevance of sympathetic nervous system activation in obesity and metabolic syndrome. J. Diabetes Res. 2015, 341583 (2015).
Bougneres, P. et al. In vivo resistance of lipolysis to epinephrine. A new feature of childhood onset obesity. J. Clin. Invest. 99, 2568–2573 (1997).
Horowitz, J. F. & Klein, S. Whole body and abdominal lipolytic sensitivity to epinephrine is suppressed in upper body obese women. Am. J. Physiol. Endocrinol. Metab. 278, E1144–E1152 (2000).
Heinonen, S. et al. Impaired mitochondrial biogenesis in adipose tissue in acquired obesity. Diabetes 64, 3135–3145 (2015).
Guo, T. et al. Adipocyte ALK7 links nutrient overload to catecholamine resistance in obesity. eLife 3, e03245 (2014).
Buettner, C. Is hyperinsulinemia required to develop overeating-induced obesity? Cell Metab. 16, 691–692 (2012).
Mehran, A. E. et al. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab. 16, 723–737 (2012).
Collins, S., Daniel, K. W. & Rohlfs, E. M. Depressed expression of adipocyte beta-adrenergic receptors is a common feature of congenital and diet-induced obesity in rodents. Int. J. Obes. Relat. Metab. Disord. 23, 669–677 (1999).
Surwit, R. S., Dixon, T. M., Petro, A. E., Daniel, K. W. & Collins, S. Diazoxide restores beta3-adrenergic receptor function in diet-induced obesity and diabetes. Endocrinology 141, 3630–3637 (2000).
Rahmouni, K. et al. Hypothalamic PI3K and MAPK differentially mediate regional sympathetic activation to insulin. J. Clin. Invest. 114, 652–658 (2004).
Muntzel, M. S., Morgan, D. A., Mark, A. L. & Johnson, A. K. Intracerebroventricular insulin produces nonuniform regional increases in sympathetic nerve activity. Am. J. Physiol. 267, R1350–R1355 (1994).
Komohara, Y., Fujiwara, Y., Ohnishi, K., Shiraishi, D. & Takeya, M. Contribution of macrophage polarization to metabolic diseases. J. Atheroscler. Thromb. 23, 10–17 (2016).
Olefsky, J. M. & Glass, C. K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 72, 219–246 (2010).
Lumeng, C. N., Bodzin, J. L. & Saltiel, A. R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 (2007).
Aron-Wisnewsky, J. et al. Human adipose tissue macrophages: m1 and m2 cell surface markers in subcutaneous and omental depots and after weight loss. J. Clin. Endocrinol. Metab. 94, 4619–4623 (2009).
Patsouris, D. et al. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 8, 301–309 (2008).
Oh, D. Y. et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142, 687–698 (2010).
Grailer, J. J., Haggadone, M. D., Sarma, J. V., Zetoune, F. S. & Ward, P. A. Induction of M2 regulatory macrophages through the beta2-adrenergic receptor with protection during endotoxemia and acute lung injury. J. Innate Immun. 6, 607–618 (2014).
Czech, M. P. Macrophages dispose of catecholamines in adipose tissue. Nat. Med. 23, 1255–1257 (2017).
Cao, Y. Angiogenesis and vascular functions in modulation of obesity, adipose metabolism, and insulin sensitivity. Cell Metab. 18, 478–489 (2013).
Garg, J. et al. Catecholamines facilitate VEGF-dependent angiogenesis via beta2-adrenoceptor-induced Epac1 and PKA activation. Oncotarget 8, 44732–44748 (2017).
Domigan, C. K. et al. Autocrine VEGF maintains endothelial survival through regulation of metabolism and autophagy. J. Cell Sci. 128, 2236–2248 (2015).
Lee, S. et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell 130, 691–703 (2007).
Marko, S. B. & Damon, D. H. VEGF promotes vascular sympathetic innervation. Am. J. Physiol. Heart Circ. Physiol. 294, H2646–H2652 (2008).
Long, J. B., Jay, S. M., Segal, S. S. & Madri, J. A. VEGF-A and semaphorin3A: modulators of vascular sympathetic innervation. Dev. Biol. 334, 119–132 (2009).
Robciuc, M. R. et al. VEGFB/VEGFR1-induced expansion of adipose vasculature counteracts obesity and related metabolic complications. Cell Metab. 23, 712–724 (2016).
During, M. J. et al. Adipose VEGF links the white-to-brown fat switch with environmental, genetic, and pharmacological stimuli in male mice. Endocrinology 156, 2059–2073 (2015).
Kim, K. H. et al. Intermittent fasting promotes adipose thermogenesis and metabolic homeostasis via VEGF-mediated alternative activation of macrophage. Cell Res. 27, 1309–1326 (2017).
Xue, Y. et al. Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell Metab. 9, 99–109 (2009).
Sun, K. et al. Brown adipose tissue derived VEGF-A modulates cold tolerance and energy expenditure. Mol. Metab. 3, 474–483 (2014).
Zhao, Y. et al. Transient overexpression of VEGF-A in adipose tissue promotes energy expenditure via activation of the sympathetic nervous system. Mol. Cell. Biol. 38, e00242–18 (2018).
Jiang, Y., Berry, D. C. & Graff, J. M. Distinct cellular and molecular mechanisms for beta3 adrenergic receptor-induced beige adipocyte formation. eLife 6, e30329 (2017).
Ueta, C. B. et al. beta(1) Adrenergic receptor is key to cold- and diet-induced thermogenesis in mice. J. Endocrinol. 214, 359–365 (2012).
Shah, S. H. et al. Neuropeptide Y gene polymorphisms confer risk of early-onset atherosclerosis. PLOS Genet. 5, e1000318 (2009).
Sung, C. P., Arleth, A. J. & Feuerstein, G. Z. Neuropeptide Y upregulates the adhesiveness of human endothelial cells for leukocytes. Circ. Res. 68, 314–318 (1991).
Claxson, A. et al. The anti-inflammatory effects of D-myo-inositol-1.2.6-trisphosphate (PP56) on animal models of inflammation. Agents Act. 29, 68–70 (1990).
Singer, K. et al. Neuropeptide Y is produced by adipose tissue macrophages and regulates obesity-induced inflammation. PLOS ONE 8, e57929 (2013).
Pandolfi, J. et al. Purinergic signaling modulates human visceral adipose inflammatory responses: implications in metabolically unhealthy obesity. J. Leukoc. Biol. 97, 941–949 (2015).
Eltzschig, H. K., Sitkovsky, M. V. & Robson, S. C. Purinergic signaling during inflammation. N. Engl. J. Med. 367, 2322–2333 (2012).
Jo, E. K., Kim, J. K., Shin, D. M. & Sasakawa, C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell. Mol. Immunol. 13, 148–159 (2016).
Enjyoji, K. et al. Deletion of cd39/entpd1 results in hepatic insulin resistance. Diabetes 57, 2311–2320 (2008).
Chen, Z. et al. Nrg4 promotes fuel oxidation and a healthy adipokine profile to ameliorate diet-induced metabolic disorders. Mol. Metab. 6, 863–872 (2017).
Jeong, J. H., Chang, J. S. & Jo, Y. H. Intracellular glycolysis in brown adipose tissue is essential for optogenetically induced nonshivering thermogenesis in mice. Sci. Rep. 8, 6672 (2018).
Akhmedov, D. et al. Gs-DREADD knock-in mice for tissue-specific, temporal stimulation of cyclic AMP signaling. Mol. Cell. Biol. 37, e00584–16 (2017).
Rojas, J. M. & Schwartz, M. W. Control of hepatic glucose metabolism by islet and brain. Diabetes Obes. Metab. 16 (Suppl. 1), 33–40 (2014).
Cantu, R. C. & Goodman, H. M. Effects of denervation and fasting on white adipose tissue. Am. J. Physiol. 212, 207–212 (1967).
Bartness, T. J., Shrestha, Y. B., Vaughan, C. H., Schwartz, G. J. & Song, C. K. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol. Cell. Endocrinol. 318, 34–43 (2010).
Acknowledgements
Work from the laboratory of M.P.C. discussed in this Review was supported by NIH grants DK30898 and DK103047 to M.P.C.
Reviewer information
Nature Reviews Endocrinology thanks K. Rahmouni, and other anonymous reviewers, for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
A.G., F.H., A.H.B. and M.P.C. contributed equally to the discussion of content, researching data for the article, writing the article and reviewing and editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guilherme, A., Henriques, F., Bedard, A.H. et al. Molecular pathways linking adipose innervation to insulin action in obesity and diabetes mellitus. Nat Rev Endocrinol 15, 207–225 (2019). https://doi.org/10.1038/s41574-019-0165-y
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-019-0165-y