Abstract
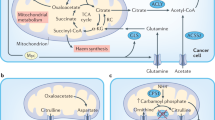
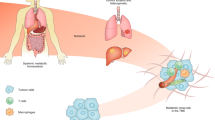
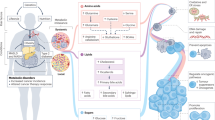
Metabolic reprogramming of cancer cells and the tumour microenvironment are pivotal characteristics of cancers, and studying these processes offer insights and avenues for cancer diagnostics and therapeutics. Recent advancements have underscored the impact of host systemic features, termed macroenvironment, on facilitating cancer progression. During tumorigenesis, these inherent features of the host, such as germline genetics, immune profile and the metabolic status, influence how the body responds to cancer. In parallel, as cancer grows, it induces systemic effects beyond the primary tumour site and affects the macroenvironment, for example, through inflammation, the metabolic end-stage syndrome of cachexia, and metabolic dysregulation. Therefore, understanding the intricate metabolic interplay between the tumour and the host is a growing frontier in advancing cancer diagnosis and therapy. In this Review, we explore the specific contribution of the metabolic fitness of the host to cancer initiation, progression and response to therapy. We then delineate the complex metabolic crosstalk between the tumour, the microenvironment and the host, which promotes disease progression to metastasis and cachexia. The metabolic relationships among the host, cancer pathogenesis and the consequent responsive systemic manifestations during cancer progression provide new perspectives for mechanistic cancer therapy and improved management of patients with cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bergers, G. & Fendt, S. M. The metabolism of cancer cells during metastasis. Nat. Rev. Cancer 21, 162–180 (2021).
Berenblum, I. The cocarcinogenic action of croton resin. Cancer Res. 1, 44–48 (1941).
Kawai, T., Autieri, M. V. & Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 320, C375–C391 (2021).
Kaaks, R. & Lukanova, A. Energy balance and cancer: the role of insulin and insulin-like growth factor-I. Proc. Nutr. Soc. 60, 91–106 (2001).
Morigny, P., Boucher, J., Arner, P. & Langin, D. Lipid and glucose metabolism in white adipocytes: pathways, dysfunction and therapeutics. Nat. Rev. Endocrinol. 17, 276–295 (2021).
Cox, A. J., West, N. P. & Cripps, A. W. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 3, 207–215 (2015).
Van Hul, M. & Cani, P. D. The gut microbiota in obesity and weight management: microbes as friends or foe? Nat. Rev. Endocrinol. 19, 258–271 (2023).
Quail, D. F. & Dannenberg, A. J. The obese adipose tissue microenvironment in cancer development and progression. Nat. Rev. Endocrinol. 15, 139–154 (2019).
Maguire, O. A. et al. Creatine-mediated crosstalk between adipocytes and cancer cells regulates obesity-driven breast cancer. Cell Metab. 33, 499–512.e6 (2021).
Kershaw, E. E. & Flier, J. S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 89, 2548–2556 (2004).
Galic, S., Oakhill, J. S. & Steinberg, G. R. Adipose tissue as an endocrine organ. Mol. Cell Endocrinol. 316, 129–139 (2010).
Wiseman, H. & Halliwell, B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem. J. 313, 17–29 (1996).
Ohnishi, S. et al. DNA damage in inflammation-related carcinogenesis and cancer stem cells. Oxid. Med. Cell Longev. 2013, 387014 (2013).
Hotamisligil, G. S., Shargill, N. S. & Spiegelman, B. M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259, 87–91 (1993).
Feinstein, R., Kanety, H., Papa, M. Z., Lunenfeld, B. & Karasik, A. Tumor necrosis factor-alpha suppresses insulin-induced tyrosine phosphorylation of insulin receptor and its substrates. J. Biol. Chem. 268, 26055–26058 (1993).
Cowey, S. & Hardy, R. W. The metabolic syndrome: a high-risk state for cancer? Am. J. Pathol. 169, 1505–1522 (2006).
Schwingshackl, L., Schwedhelm, C., Galbete, C. & Hoffmann, G. Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta-analysis. Nutrients https://doi.org/10.3390/nu9101063 (2017).
Wang, T., Masedunskas, A., Willett, W. C. & Fontana, L. Vegetarian and vegan diets: benefits and drawbacks. Eur. Heart J. 44, 3423–3439 (2023).
Abe, C. et al. A longitudinal association between the Traditional Japanese Diet Score and incidence and mortality of breast cancer-an ecological study. Eur. J. Clin. Nutr. 75, 929–936 (2021).
Takasu, A. et al. Daily diet and nutrition risk factors for gastric cancer incidence in a Japanese population. Gut Liver 18, 602–610 (2024).
Huang, J. et al. Association between plant and animal protein intake and overall and cause-specific mortality. JAMA Intern. Med. 180, 1173–1184 (2020).
Farvid, M. S. et al. Consumption of red meat and processed meat and cancer incidence: a systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 36, 937–951 (2021).
Dyar, K. A. et al. Atlas of circadian metabolism reveals system-wide coordination and communication between clocks. Cell 174, 1571–1585.e11 (2018). This work describes comprehensive maps of circadian metabolism across mouse tissues in the context of systemic energy balance and under chronic nutrient stress (high fat diet), revealing a key role of circadian rhythm in tissue nutrient availability.
Sullivan, M. R. et al. Quantification of microenvironmental metabolites in murine cancers reveals determinants of tumor nutrient availability. eLife https://doi.org/10.7554/eLife.44235 (2019).
Allen, A. M., Hicks, S. B., Mara, K. C., Larson, J. J. & Therneau, T. M. The risk of incident extrahepatic cancers is higher in non-alcoholic fatty liver disease than obesity — a longitudinal cohort study. J. Hepatol. 71, 1229–1236 (2019).
Mahale, P. et al. Hepatitis C virus infection and the risk of cancer among elderly US adults: a registry-based case-control study. Cancer 123, 1202–1211 (2017).
Pol, S., Vallet-Pichard, A. & Hermine, O. Extrahepatic cancers and chronic HCV infection. Nat. Rev. Gastroenterol. Hepatol. 15, 283–290 (2018).
Sabbagh, C. et al. Management of colon cancer in patients with cirrhosis: a review. Surg. Oncol. 24, 187–193 (2015).
Yoshimoto, S. et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499, 97–101 (2013).
Tilg, H. & Moschen, A. R. Mechanisms behind the link between obesity and gastrointestinal cancers. Best Pract. Res. Clin. Gastroenterol. 28, 599–610 (2014).
Goldman, O. et al. Early infiltration of innate immune cells to the liver depletes HNF4α and promotes extrahepatic carcinogenesis. Cancer Discov. https://doi.org/10.1158/2159-8290.CD-22-1062 (2023).
Kut, E. & Menekse, S. Prognostic significance of pretreatment albumin-bilirubin (ALBI) grade and platelet-albumin-bilirubin (PALBI) grade in patients with small cell lung cancer. Sci. Rep. 14, 1371 (2024).
Drapela, S., Ilter, D. & Gomes, A. P. Metabolic reprogramming: a bridge between aging and tumorigenesis. Mol. Oncol. 16, 3295–3318 (2022).
Ross, J. M. et al. High brain lactate is a hallmark of aging and caused by a shift in the lactate dehydrogenase A/B ratio. Proc. Natl Acad. Sci. USA 107, 20087–20092 (2010).
Fang, E. F. et al. NAD+ in aging: molecular mechanisms and translational implications. Trends Mol. Med. 23, 899–916 (2017).
Connor, K. M. et al. Understanding metabolic changes in aging bone marrow. Exp. Hematol. Oncol. 7, 13 (2018).
Ding, J. et al. A metabolome atlas of the aging mouse brain. Nat. Commun. 12, 6021 (2021). This work presents a comprehensive metabolome atlas of the ageing mouse brain, highlighting region-specific and age-specific metabolic changes, providing a valuable resource for future studies.
Tchkonia, T. et al. Fat tissue, aging, and cellular senescence. Aging Cell 9, 667–684 (2010).
Palmer, A. K. & Jensen, M. D. Metabolic changes in aging humans: current evidence and therapeutic strategies. J. Clin. Invest. https://doi.org/10.1172/JCI158451 (2022).
Salzer, M. C. et al. Identity noise and adipogenic traits characterize dermal fibroblast aging. Cell 175, 1575–1590.e22 (2018).
Alicea, G. M. et al. Changes in aged fibroblast lipid metabolism induce age-dependent melanoma cell resistance to targeted therapy via the fatty acid transporter FATP2. Cancer Discov. 10, 1282–1295 (2020).
Baker, D. J. et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530, 184–189 (2016).
Childs, B. G. et al. Senescent cells: an emerging target for diseases of ageing. Nat. Rev. Drug Discov. 16, 718–735 (2017).
Guo, J. et al. Aging and aging-related diseases: from molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 7, 391 (2022).
Nandy, A. et al. Altered osteoblast metabolism with aging results in lipid accumulation and oxidative stress mediated bone loss. Aging Dis. 15, 767–786 (2024).
Zhang, W. et al. Nomogram predicts risk and prognostic factors for bone metastasis of pancreatic cancer: a population-based analysis. Front. Endocrinol. 12, 752176 (2021).
Purushotham, A. et al. Age at diagnosis and distant metastasis in breast cancer — a surprising inverse relationship. Eur. J. Cancer 50, 1697–1705 (2014).
Hojman, P., Gehl, J., Christensen, J. F. & Pedersen, B. K. Molecular mechanisms linking exercise to cancer prevention and treatment. Cell Metab. 27, 10–21 (2018).
Laurens, C., de Glisezinski, I., Larrouy, D., Harant, I. & Moro, C. Influence of acute and chronic exercise on abdominal fat lipolysis: an update. Front. Physiol. 11, 575363 (2020).
Nigro, P. et al. Exercise training remodels inguinal white adipose tissue through adaptations in innervation, vascularization, and the extracellular matrix. Cell Rep. 42, 112392 (2023).
Lin, T. C. & Hsiao, M. Leptin and cancer: updated functional roles in carcinogenesis, therapeutic niches, and developments. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22062870 (2021).
Navarro-Ledesma, S., Hamed-Hamed, D., Gonzalez-Munoz, A. & Pruimboom, L. Physical activity, insulin resistance and cancer: a systematic review. Cancers https://doi.org/10.3390/cancers16030656 (2024).
Souza, J. et al. Physical-exercise-induced antioxidant effects on the brain and skeletal muscle. Antioxidants https://doi.org/10.3390/antiox11050826 (2022).
Gomez-Cabrera, M. C., Domenech, E. & Vina, J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic. Biol. Med. 44, 126–131 (2008).
Scarfo, G., Daniele, S. & Franzoni, F. Antioxidant capability and physical exercise in neurobiology: a focus in neurodegeneration. Antioxidants https://doi.org/10.3390/antiox10020250 (2021).
Scarfo, G. et al. Regular exercise delays microvascular endothelial dysfunction by regulating antioxidant capacity and cellular metabolism. Sci. Rep. 13, 17671 (2023).
Mousavi, S. R., Jafari, M., Rezaei, S., Agha-Alinejad, H. & Sobhani, V. Evaluation of the effects of different intensities of forced running wheel exercise on oxidative stress biomarkers in muscle, liver and serum of untrained rats. Lab. Anim. 49, 119–125 (2020).
Liskiewicz, A. et al. Physical activity reduces anxiety and regulates brain fatty acid synthesis. Mol. Brain 13, 62 (2020).
MoTrPAC Study Group, Lead Analysts & MoTrPAC Study GroupTemporal dynamics of the multi-omic response to endurance exercise training. Nature 629, 174–183 (2024).
Franczyk, B., Gluba-Brzozka, A., Cialkowska-Rysz, A., Lawinski, J. & Rysz, J. The impact of aerobic exercise on HDL quantity and quality: a narrative review. Int. J. Mol. Sci. https://doi.org/10.3390/ijms24054653 (2023).
Thomas, R., Kenfield, S. A., Yanagisawa, Y. & Newton, R. U. Why exercise has a crucial role in cancer prevention, risk reduction and improved outcomes. Br. Med. Bull. 139, 100–119 (2021).
Sheinboim, D. et al. An exercise-induced metabolic shield in distant organs blocks cancer progression and metastatic dissemination. Cancer Res. 82, 4164–4178 (2022). This work explores an interesting angle of the field exploring how exercise can help cancer outcomes both before and after diagnosis.
Lu, M. et al. Exercise inhibits tumor growth and central carbon metabolism in patient-derived xenograft models of colorectal cancer. Cancer Metab. 6, 14 (2018).
Pedersen, L. et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. 23, 554–562 (2016).
Siqueira, I. R., Batabyal, R. A., Freishtat, R. & Cechinel, L. R. Potential involvement of circulating extracellular vesicles and particles on exercise effects in malignancies. Front. Endocrinol. 14, 1121390 (2023).
Kurz, E. et al. Exercise-induced engagement of the IL-15/IL-15Rα axis promotes anti-tumor immunity in pancreatic cancer. Cancer Cell 40, 720–737.e5 (2022).
McTiernan, A. Mechanisms linking physical activity with cancer. Nat. Rev. Cancer 8, 205–211 (2008).
Schmitz, K. H. et al. Exercise is medicine in oncology: engaging clinicians to help patients move through cancer. CA Cancer J. Clin. 69, 468–484 (2019).
Guillerey, C., Huntington, N. D. & Smyth, M. J. Targeting natural killer cells in cancer immunotherapy. Nat. Immunol. 17, 1025–1036 (2016).
Waldmann, T. A. Interleukin-15 in the treatment of cancer. Expert. Rev. Clin. Immunol. 10, 1689–1701 (2014).
Steele, N. et al. A phase 1 trial of recombinant human IL-21 in combination with cetuximab in patients with metastatic colorectal cancer. Br. J. Cancer 106, 793–798 (2012).
Guo, Y., Xu, T., Chai, Y. & Chen, F. TGF-β signaling in progression of oral cancer. Int. J. Mol. Sci. https://doi.org/10.3390/ijms241210263 (2023).
Lawrence, M. S. et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 505, 495–501 (2014).
Hoxhaj, G. & Manning, B. D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 20, 74–88 (2020).
Yu, M. et al. Development and safety of PI3K inhibitors in cancer. Arch. Toxicol. 97, 635–650 (2023).
Mayer, I. A. & Arteaga, C. L. The PI3K/AKT pathway as a target for cancer treatment. Annu. Rev. Med. 67, 11–28 (2016).
He, Y. et al. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target. Ther. 6, 425 (2021).
Motzer, R. J. et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer 116, 4256–4265 (2010).
Janku, F., Yap, T. A. & Meric-Bernstam, F. Targeting the PI3K pathway in cancer: are we making headway? Nat. Rev. Clin. Oncol. 15, 273–291 (2018).
Fruman, D. A. et al. The PI3K pathway in human disease. Cell 170, 605–635 (2017).
Goncalves, M. D., Hopkins, B. D. & Cantley, L. C. Phosphatidylinositol 3-kinase, growth disorders, and cancer. N. Engl. J. Med. 379, 2052–2062 (2018).
Hopkins, B. D. et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature 560, 499–503 (2018). Despite promising pre-clinical results, PI3K inhibitors showed limited effectiveness in clinical trials. This work reveals mechanistic insights of PI3K signalling reactivation in tumours upon inhibition by insulin feedback, and it demonstrates that preventing this feedback through host systemic modulations significantly improves the efficacy of PI3K inhibitors.
Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496, 238–242 (2013).
Collins, N. et al. The bone marrow protects and optimizes immunological memory during dietary restriction. Cell 178, 1088–1101.e15 (2019).
Speakman, J. R. & Mitchell, S. E. Caloric restriction. Mol. Asp. Med. 32, 159–221 (2011).
Forni, M. F. et al. Caloric restriction promotes structural and metabolic changes in the skin. Cell Rep. 20, 2678–2692 (2017).
Zhu, H. et al. Ketogenic diet for human diseases: the underlying mechanisms and potential for clinical implementations. Signal Transduct. Target. Ther. 7, 11 (2022).
Weber, D. D. et al. Ketogenic diet in the treatment of cancer — where do we stand? Mol. Metab. 33, 102–121 (2020).
Massey, K. A. & Nicolaou, A. Lipidomics of polyunsaturated-fatty-acid-derived oxygenated metabolites. Biochem. Soc. Trans. 39, 1240–1246 (2011).
Yin, H., Xu, L. & Porter, N. A. Free radical lipid peroxidation: mechanisms and analysis. Chem. Rev. 111, 5944–5972 (2011).
Nair, J. et al. High dietary omega-6 polyunsaturated fatty acids drastically increase the formation of etheno-DNA base adducts in white blood cells of female subjects. Cancer Epidemiol. Biomark. Prev. 6, 597–601 (1997).
Little, C. & O’Brien, P. J. An intracellular GSH-peroxidase with a lipid peroxide substrate. Biochem. Biophys. Res. Commun. 31, 145–150 (1968).
Yang, W. S. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331 (2014).
Oliveira, C. L. P. et al. A nutritional perspective of ketogenic diet in cancer: a narrative review. J. Acad. Nutr. Diet. 118, 668–688 (2018).
Nencioni, A., Caffa, I., Cortellino, S. & Longo, V. D. Fasting and cancer: molecular mechanisms and clinical application. Nat. Rev. Cancer 18, 707–719 (2018).
Chen, Y. et al. Metabolic intervention by low carbohydrate diet suppresses the onset and progression of neuroendocrine tumors. Cell Death Dis. 14, 597 (2023).
Lee, A. C. et al. Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. J. Biol. Chem. 274, 7936–7940 (1999).
Wei, S. J. et al. Ketogenic diet induces p53-dependent cellular senescence in multiple organs. Sci. Adv. 10, eado1463 (2024).
Su, Z., Liu, Y., Xia, Z., Rustgi, A. K. & Gu, W. An unexpected role for the ketogenic diet in triggering tumor metastasis by modulating BACH1-mediated transcription. Sci. Adv. 10, eadm9481 (2024).
Ferrer, M. et al. Ketogenic diet promotes tumor ferroptosis but induces relative corticosterone deficiency that accelerates cachexia. Cell Metab. 35, 1147–1162.e7 (2023).
Gao, X. et al. Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature 572, 397–401 (2019). This work shows that a targeted dietary manipulation of an essential amino acid can specifically affect tumour–cell metabolism and enhance the effectiveness of chemotherapy and radiation therapies, providing mechanistic evidence of the opportunities for improvements using dietary interventions in combination with current treatments.
Chaturvedi, S., Hoffman, R. M. & Bertino, J. R. Exploiting methionine restriction for cancer treatment. Biochem. Pharmacol. 154, 170–173 (2018).
Badgley, M. A. et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science 368, 85–89 (2020).
Jain, M. et al. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science 336, 1040–1044 (2012).
Maddocks, O. D. et al. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature 493, 542–546 (2013).
Maddocks, O. D. K. et al. Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature 544, 372–376 (2017). This work shows that targeted dietary manipulation of non-essential amino acids can slow tumour growth, offering a potential therapeutic approach. However, it also highlights how genetic factors such as oncogenic activation can influence the response to dietary interventions.
Xiao, F. et al. Leucine deprivation inhibits proliferation and induces apoptosis of human breast cancer cells via fatty acid synthase. Oncotarget 7, 63679–63689 (2016).
Leone, R. D. et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science 366, 1013–1021 (2019).
Knott, S. R. V. et al. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature 554, 378–381 (2018).
Ilerhunmwuwa, N. P. et al. Dietary interventions in cancer: a systematic review of all randomized controlled trials. J. Natl Cancer Inst. 116, 1026–1034 (2024).
Soldati, L. et al. The influence of diet on anti-cancer immune responsiveness. J. Transl. Med. 16, 75 (2018).
Piening, A. et al. Obesity-related T cell dysfunction impairs immunosurveillance and increases cancer risk. Nat. Commun. 15, 2835 (2024).
Dai, X. et al. Energy status dictates PD-L1 protein abundance and anti-tumor immunity to enable checkpoint blockade. Mol. Cell 81, 2317–2331.e16 (2021).
Ferrere, G. et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight https://doi.org/10.1172/jci.insight.145207 (2021).
Skrajnowska, D. & Bobrowska-Korczak, B. Role of zinc in immune system and anti-cancer defense mechanisms. Nutrients https://doi.org/10.3390/nu11102273 (2019).
Stiles, L. I., Ferrao, K. & Mehta, K. J. Role of zinc in health and disease. Clin. Exp. Med. 24, 38 (2024).
Gao, H., Dai, W., Zhao, L., Min, J. & Wang, F. The role of zinc and zinc homeostasis in macrophage function. J. Immunol. Res. 2018, 6872621 (2018).
Wessels, I., Haase, H., Engelhardt, G., Rink, L. & Uciechowski, P. Zinc deficiency induces production of the proinflammatory cytokines IL-1β and TNFα in promyeloid cells via epigenetic and redox-dependent mechanisms. J. Nutr. Biochem. 24, 289–297 (2013).
Fernandes, G. et al. Impairment of cell-mediated immunity functions by dietary zinc deficiency in mice. Proc. Natl Acad. Sci. USA 76, 457–461 (1979).
Dardenne, M., Pleau, J. M., Savino, W., Prasad, A. S. & Bach, J. F. Biochemical and biological aspects of the interaction between thymulin and zinc. Prog. Clin. Biol. Res. 380, 23–32 (1993).
Lin, L. C. et al. Effects of zinc supplementation on clinical outcomes in patients receiving radiotherapy for head and neck cancers: a double-blinded randomized study. Int. J. Radiat. Oncol. Biol. Phys. 70, 368–373 (2008).
Lin, Y. S., Lin, L. C. & Lin, S. W. Effects of zinc supplementation on the survival of patients who received concomitant chemotherapy and radiotherapy for advanced nasopharyngeal carcinoma: follow-up of a double-blind randomized study with subgroup analysis. Laryngoscope 119, 1348–1352 (2009).
Prasad, A. S. Discovery of human zinc deficiency: its impact on human health and disease. Adv. Nutr. 4, 176–190 (2013).
Mocchegiani, E. et al. Zinc, metallothioneins and immunosenescence: effect of zinc supply as nutrigenomic approach. Biogerontology 12, 455–465 (2011).
Magrone, T., Pugliese, V., Fontana, S. & Jirillo, E. Human use of Leucoselect® Phytosome® with special reference to inflammatory-allergic pathologies in frail elderly patients. Curr. Pharm. Des. 20, 1011–1019 (2014).
Saito, Y. & Soga, T. Amino acid transporters as emerging therapeutic targets in cancer. Cancer Sci. 112, 2958–2965 (2021).
Lemos, H., Huang, L., Prendergast, G. C. & Mellor, A. L. Immune control by amino acid catabolism during tumorigenesis and therapy. Nat. Rev. Cancer 19, 162–175 (2019).
Czystowska-Kuzmicz, M. et al. Small extracellular vesicles containing arginase-1 suppress T-cell responses and promote tumor growth in ovarian carcinoma. Nat. Commun. 10, 3000 (2019).
Ma, E. H. et al. Serine is an essential metabolite for effector T cell expansion. Cell Metab. 25, 345–357 (2017).
Rodriguez, P. C. et al. Regulation of T cell receptor CD3ζ chain expression by l-arginine. J. Biol. Chem. 277, 21123–21129 (2002).
Rodriguez, P. C., Quiceno, D. G. & Ochoa, A. C. l-Arginine availability regulates T-lymphocyte cell-cycle progression. Blood 109, 1568–1573 (2007).
Klysz, D. et al. Glutamine-dependent α-ketoglutarate production regulates the balance between T helper 1 cell and regulatory T cell generation. Sci. Signal. 8, ra97 (2015).
Lee, G. K. et al. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology 107, 452–460 (2002).
Munn, D. H. et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity 22, 633–642 (2005).
Narsale, A. et al. Cancer-driven changes link T cell frequency to muscle strength in people with cancer: a pilot study. J. Cachexia Sarcopenia Muscle 10, 827–843 (2019).
Bleve, A., Durante, B., Sica, A. & Consonni, F. M. Lipid metabolism and cancer immunotherapy: immunosuppressive myeloid cells at the crossroad. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21165845 (2020).
Hotamisligil, G. S. Inflammation, metaflammation and immunometabolic disorders. Nature 542, 177–185 (2017).
Duncan, R. E., Ahmadian, M., Jaworski, K., Sarkadi-Nagy, E. & Sul, H. S. Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 27, 79–101 (2007).
Luo, W., Xu, Q., Wang, Q., Wu, H. & Hua, J. Effect of modulation of PPAR-γ activity on Kupffer cells M1/M2 polarization in the development of non-alcoholic fatty liver disease. Sci. Rep. 7, 44612 (2017).
Catella, F. et al. Biosynthesis of P450 products of arachidonic acid in humans: increased formation in cardiovascular disease. Adv. Prostaglandin Thromboxane Leukot. Res. 21A, 193–196 (1991).
Biswas, S. K. Metabolic reprogramming of immune cells in cancer progression. Immunity 43, 435–449 (2015).
Huang, S. C. et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat. Immunol. 15, 846–855 (2014).
Odegaard, J. I. & Chawla, A. Alternative macrophage activation and metabolism. Annu. Rev. Pathol. 6, 275–297 (2011).
Hossain, F. et al. Inhibition of fatty acid oxidation modulates immunosuppressive functions of myeloid-derived suppressor cells and enhances cancer therapies. Cancer Immunol. Res. 3, 1236–1247 (2015).
McQuade, J. L. et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 19, 310–322 (2018). In contrast to obesity effects in other contexts of cancer, this work demonstrates that obesity is associated with improved progression-free and overall survival in male patients with metastatic melanoma treated with targeted or immune therapies, highlighting the importance of understanding the molecular mechanisms linked to obesity in the context of the hosts.
Albiges, L. et al. Body mass index and metastatic renal cell carcinoma: clinical and biological correlations. J. Clin. Oncol. 34, 3655–3663 (2016).
Wang, Z. et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 25, 141–151 (2019).
Wiig, H. & Swartz, M. A. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol. Rev. 92, 1005–1060 (2012).
Jang, C. et al. Metabolite exchange between mammalian organs quantified in pigs. Cell Metab. 30, 594–606.e3 (2019).
Hui, S. et al. Glucose feeds the TCA cycle via circulating lactate. Nature 551, 115–118 (2017).
Faubert, B. et al. Lactate metabolism in human lung tumors. Cell 171, 358–371.e9 (2017). This work reveals for the first time in infused non-small-cell lung cancer patients that lactate, not just glucose, serves as a major fuel source for the tricarboxylic acid cycle, highlighting a critical role for lactate in tumour metabolism beyond a waste metabolic product.
Tran, D. H. et al. De novo and salvage purine synthesis pathways across tissues and tumors. Cell 187, 3602–3618.e20 (2024).
Wu, Z. et al. Electron transport chain inhibition increases cellular dependence on purine transport and salvage. Cell Metab. 36, 1504–1520.e9 (2024).
Pavlides, S. et al. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 8, 3984–4001 (2009).
Andersen, M. K. et al. Spatial differentiation of metabolism in prostate cancer tissue by MALDI-TOF MSI. Cancer Metab. 9, 9 (2021).
Sun, C., Wang, F., Zhang, Y., Yu, J. & Wang, X. Mass spectrometry imaging-based metabolomics to visualize the spatially resolved reprogramming of carnitine metabolism in breast cancer. Theranostics 10, 7070–7082 (2020).
Kamphorst, J. J. et al. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 75, 544–553 (2015). This work reveals a mechanism by which pancreatic tumours can take advantage of the host by scavenging extracellular proteins for amino acids, to sustain growth in poorly vascularized cancer.
Sousa, C. M. et al. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature 536, 479–483 (2016).
Auciello, F. R. et al. A stromal lysolipid-autotaxin signaling axis promotes pancreatic tumor progression. Cancer Discov. 9, 617–627 (2019).
Li, F. et al. FBP1 loss disrupts liver metabolism and promotes tumorigenesis through a hepatic stellate cell senescence secretome. Nat. Cell Biol. 22, 728–739 (2020).
Martini, T., Naef, F. & Tchorz, J. S. Spatiotemporal metabolic liver zonation and consequences on pathophysiology. Annu. Rev. Pathol. 18, 439–466 (2023).
Coulouarn, C. et al. Hepatocyte-stellate cell cross-talk in the liver engenders a permissive inflammatory microenvironment that drives progression in hepatocellular carcinoma. Cancer Res. 72, 2533–2542 (2012).
Sherman, M. H. Stellate cells in tissue repair, inflammation, and cancer. Annu. Rev. Cell Dev. Biol. 34, 333–355 (2018).
Lyssiotis, C. A. & Kimmelman, A. C. Metabolic interactions in the tumor microenvironment. Trends Cell Biol. 27, 863–875 (2017).
Kerk, S. A. et al. Metabolic requirement for GOT2 in pancreatic cancer depends on environmental context. eLife https://doi.org/10.7554/eLife.73245 (2022).
Attane, C. et al. Human bone marrow is comprised of adipocytes with specific lipid metabolism. Cell Rep. 30, 949–958.e6 (2020).
Panaroni, C. et al. Multiple myeloma cells induce lipolysis in adipocytes and uptake fatty acids through fatty acid transporter proteins. Blood 139, 876–888 (2022).
Shafat, M. S. et al. Leukemic blasts program bone marrow adipocytes to generate a protumoral microenvironment. Blood 129, 1320–1332 (2017).
Kumar, B. et al. Exosomes-driven lipolysis and bone marrow niche remodeling supports leukemia expansion. Haematologica 106, 1484–1488 (2020).
Motohara, T. et al. An evolving story of the metastatic voyage of ovarian cancer cells: cellular and molecular orchestration of the adipose-rich metastatic microenvironment. Oncogene 38, 2885–2898 (2019).
Nieman, K. M. et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 17, 1498–1503 (2011).
Ladanyi, A. et al. Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene 37, 2285–2301 (2018).
Mukherjee, A. et al. Adipocytes reprogram cancer cell metabolism by diverting glucose towards glycerol-3-phosphate thereby promoting metastasis. Nat. Metab. 5, 1563–1577 (2023).
Wang, Y. Y. et al. Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight 2, e87489 (2017).
Rossi, M. et al. PHGDH heterogeneity potentiates cancer cell dissemination and metastasis. Nature 605, 747–753 (2022).
Tasdogan, A. et al. Metabolic heterogeneity confers differences in melanoma metastatic potential. Nature 577, 115–120 (2020).
Gomes, A. P. et al. Altered propionate metabolism contributes to tumour progression and aggressiveness. Nat. Metab. 4, 435–443 (2022).
Mashimo, T. et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell 159, 1603–1614 (2014).
Neman, J. et al. Human breast cancer metastases to the brain display GABAergic properties in the neural niche. Proc. Natl Acad. Sci. USA 111, 984–989 (2014).
Chen, J. et al. Gain of glucose-independent growth upon metastasis of breast cancer cells to the brain. Cancer Res. 75, 554–565 (2015).
Ngo, B. et al. Limited environmental serine and glycine confer brain metastasis sensitivity to PHGDH inhibition. Cancer Discov. 10, 1352–1373 (2020).
Ferraro, G. B. et al. Fatty acid synthesis is required for breast cancer brain metastasis. Nat. Cancer 2, 414–428 (2021).
Zou, Y. et al. Polyunsaturated fatty acids from astrocytes activate PPARγ signaling in cancer cells to promote brain metastasis. Cancer Discov. 9, 1720–1735 (2019).
Parida, P. K. et al. Limiting mitochondrial plasticity by targeting DRP1 induces metabolic reprogramming and reduces breast cancer brain metastases. Nat. Cancer 4, 893–907 (2023).
Jin, X. et al. A metastasis map of human cancer cell lines. Nature 588, 331–336 (2020).
Savino, A. M. et al. Metabolic adaptation of acute lymphoblastic leukemia to the central nervous system microenvironment is dependent on stearoyl CoA desaturase. Nat. Cancer 1, 998–1009 (2020).
Schwaiger-Haber, M. et al. Using mass spectrometry imaging to map fluxes quantitatively in the tumor ecosystem. Nat. Commun. 14, 2876 (2023).
LeBleu, V. S. et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat. Cell Biol. 16, 992–1003 (2014).
Ilter, D. et al. NADK-mediated de novo NADP(H) synthesis is a metabolic adaptation essential for breast cancer metastasis. Redox Biol. 61, 102627 (2023).
Zhang, Y. et al. G6PD-mediated increase in de novo NADP+ biosynthesis promotes antioxidant defense and tumor metastasis. Sci. Adv. 8, eabo0404 (2022).
Altea-Manzano, P. et al. A palmitate-rich metastatic niche enables metastasis growth via p65 acetylation resulting in pro-metastatic NF-κB signaling. Nat. Cancer 4, 344–364 (2023).
Dupuy, F. et al. PDK1-dependent metabolic reprogramming dictates metastatic potential in breast cancer. Cell Metab. 22, 577–589 (2015).
Kietzmann, T. Metabolic zonation of the liver: the oxygen gradient revisited. Redox Biol. 11, 622–630 (2017).
Bu, P. et al. Aldolase B-mediated fructose metabolism drives metabolic reprogramming of colon cancer liver metastasis. Cell Metab. 27, 1249–1262.e4 (2018).
Yamaguchi, N. et al. PCK1 and DHODH drive colorectal cancer liver metastatic colonization and hypoxic growth by promoting nucleotide synthesis. eLife https://doi.org/10.7554/eLife.52135 (2019).
Wu, Z. et al. TPO-induced metabolic reprogramming drives liver metastasis of colorectal cancer CD110+ tumor-initiating cells. Cell Stem Cell 17, 47–59 (2015).
Zhang, L. et al. Creatine promotes cancer metastasis through activation of Smad2/3. Cell Metab. 33, 1111–1123.e4 (2021).
Loo, J. M. et al. Extracellular metabolic energetics can promote cancer progression. Cell 160, 393–406 (2015).
Journo, S. et al. Genomic alterations drive metastases formation in pancreatic ductal adenocarcinoma cancer: deciphering the role of CDKN2A and CDKN2B in mediating liver tropism. Oncogene 41, 1468–1481 (2022).
Choi, I. A., Umemoto, A., Mizuno, M. & Park-Min, K.-H. Bone metabolism — an underappreciated player. npj Metab. Health Dis. 2, 12 (2024).
Tandon, M., Othman, A. H., Winogradzki, M. & Pratap, J. Bone metastatic breast cancer cells display downregulation of PKC-ζ with enhanced glutamine metabolism. Gene 775, 145419 (2021).
Krzeszinski, J. Y. et al. Lipid osteoclastokines regulate breast cancer bone metastasis. Endocrinology 158, 477–489 (2017).
Whitburn, J. et al. Metabolic profiling of prostate cancer in skeletal microenvironments identifies G6PD as a key mediator of growth and survival. Sci. Adv. 8, eabf9096 (2022).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Narsale, A. A. & Carson, J. A. Role of interleukin-6 in cachexia: therapeutic implications. Curr. Opin. Support. Palliat. Care 8, 321–327 (2014).
Fujiwara, Y. et al. Indoleamine 2,3-dioxygenase (IDO) inhibitors and cancer immunotherapy. Cancer Treat. Rev. 110, 102461 (2022).
Munn, D. H. & Mellor, A. L. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 34, 137–143 (2013).
Lercher, A., Baazim, H. & Bergthaler, A. Systemic immunometabolism: challenges and opportunities. Immunity 53, 496–509 (2020).
Dang, Q. et al. Cancer immunometabolism: advent, challenges, and perspective. Mol. Cancer 23, 72 (2024).
Wang, Y. et al. Pre-metastatic niche: formation, characteristics and therapeutic implication. Signal Transduct. Target. Ther. 9, 236 (2024).
Fong, M. Y. et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 17, 183–194 (2015). This report shows that metabolites (glucose in this case) can be altered during the formation of the pre-metastatic niche, facilitating metastasis by altering the metabolism of local cells which increases nutrient availability to disseminated cancer cells in the niche.
Li, P. et al. Lung mesenchymal cells elicit lipid storage in neutrophils that fuel breast cancer lung metastasis. Nat. Immunol. 21, 1444–1455 (2020).
Gong, Z. et al. Lipid-laden lung mesenchymal cells foster breast cancer metastasis via metabolic reprogramming of tumor cells and natural killer cells. Cell Metab. 34, 1960–1976.e9 (2022).
Kuhlmann-Hogan, A. et al. EGFR-driven lung adenocarcinomas co-opt alveolar macrophage metabolism and function to support EGFR signaling and growth. Cancer Discov. 14, 524–545 (2024).
Ganguly, K. & Kimmelman, A. C. Reprogramming of tissue metabolism during cancer metastasis. Trends Cancer 9, 461–471 (2023).
Fearon, K. et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 12, 489–495 (2011).
Dhanapal, R., Saraswathi, T. & Rajkumar, N. Cancer cachexia. J. Oral Maxillofac. Pathol. 15, 257–260 (2011).
von Haehling, S., Anker, M. S. & Anker, S. D. Prevalence and clinical impact of cachexia in chronic illness in Europe, USA, and Japan: facts and numbers update 2016. J. Cachexia Sarcopenia Muscle 7, 507–509 (2016).
Baracos, V. E., Martin, L., Korc, M., Guttridge, D. C. & Fearon, K. C. H. Cancer-associated cachexia. Nat. Rev. Dis. Prim. 4, 17105 (2018).
Porporato, P. E. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis 5, e200 (2016).
Babic, A. et al. Adipose tissue and skeletal muscle wasting precede clinical diagnosis of pancreatic cancer. Nat. Commun. 14, 4317 (2023).
Douglas, R. G. & Shaw, J. H. Metabolic effects of cancer. Br. J. Surg. 77, 246–254 (1990).
Donohoe, C. L., Ryan, A. M. & Reynolds, J. V. Cancer cachexia: mechanisms and clinical implications. Gastroenterol. Res. Pract. 2011, 601434 (2011).
Fonseca, G., Farkas, J., Dora, E., von Haehling, S. & Lainscak, M. Cancer cachexia and related metabolic dysfunction. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21072321 (2020).
Flint, T. R. et al. Tumor-induced IL-6 reprograms host metabolism to suppress anti-tumor immunity. Cell Metab. 24, 672–684 (2016).
Goncalves, M. D. et al. Fenofibrate prevents skeletal muscle loss in mice with lung cancer. Proc. Natl Acad. Sci. USA 115, E743–E752 (2018).
Petruzzelli, M. & Wagner, E. F. Mechanisms of metabolic dysfunction in cancer-associated cachexia. Genes Dev. 30, 489–501 (2016).
O’Connell, T. M. et al. Metabolic biomarkers for the early detection of cancer cachexia. Front. Cell Dev. Biol. 9, 720096 (2021).
Wang, G. et al. Tumour extracellular vesicles and particles induce liver metabolic dysfunction. Nature 618, 374–382 (2023).
Austin, J. & Marks, D. Hormonal regulators of appetite. Int. J. Pediatr. Endocrinol. 2009, 141753 (2009).
Yoo, E. S., Yu, J. & Sohn, J. W. Neuroendocrine control of appetite and metabolism. Exp. Mol. Med. 53, 505–516 (2021).
Kim, K. S., Seeley, R. J. & Sandoval, D. A. Signalling from the periphery to the brain that regulates energy homeostasis. Nat. Rev. Neurosci. 19, 185–196 (2018).
Lockhart, S. M., Saudek, V. & O’Rahilly, S. GDF15: a hormone conveying somatic distress to the brain. Endocr. Rev. https://doi.org/10.1210/endrev/bnaa007 (2020).
Ni, J. & Zhang, L. Cancer cachexia: definition, staging, and emerging treatments. Cancer Manag. Res. 12, 5597–5605 (2020).
Hart, B. L. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 12, 123–137 (1988).
Fantino, M. & Wieteska, L. Evidence for a direct central anorectic effect of tumor-necrosis-factor-alpha in the rat. Physiol. Behav. 53, 477–483 (1993).
Scarlett, J. M. et al. Regulation of central melanocortin signaling by interleukin-1β. Endocrinology 148, 4217–4225 (2007).
Plata-Salaman, C. R., Sonti, G., Borkoski, J. P., Wilson, C. D. & French-Mullen, J. M. B. Anorexia induced by chronic central administration of cytokines at estimated pathophysiological concentrations. Physiol. Behav. 60, 867–875 (1996).
Kapas, L. & Krueger, J. M. Tumor necrosis factor-beta induces sleep, fever, and anorexia. Am. J. Physiol. 263, R703–R707 (1992).
Sonti, G., Ilyin, S. E. & Plata-Salaman, C. R. Anorexia induced by cytokine interactions at pathophysiological concentrations. Am. J. Physiol. 270, R1394–R1402 (1996).
Gayle, D., Ilyin, S. E. & Plata-Salaman, C. R. Central nervous system IL-1β system and neuropeptide Y mRNAs during IL-1β-induced anorexia in rats. Brain Res. Bull. 44, 311–317 (1997).
Wu, Q., Chen, J., Hua, T. & Cai, J. Alpha-melanocyte-stimulating hormone-mediated appetite regulation in the central nervous system. Neuroendocrinology 113, 885–904 (2023).
Cernackova, A., Tillinger, A., Bizik, J., Mravec, B. & Horvathova, L. Dynamics of cachexia-associated inflammatory changes in the brain accompanying intra-abdominal fibrosarcoma growth in Wistar rats. J. Neuroimmunol. 376, 578033 (2023).
Sun, Q. et al. Area postrema neurons mediate interleukin-6 function in cancer cachexia. Nat. Commun. 15, 4682 (2024). This work highlights the multisystemic nature of the mechanism leading to cachexia pathogenicity, involving central nervous system dysregulation by circulating IL-6.
Dodson, S. et al. Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annu. Rev. Med. 62, 265–279 (2011).
Gullett, N. P., Mazurak, V. C., Hebbar, G. & Ziegler, T. R. Nutritional interventions for cancer-induced cachexia. Curr. Probl. Cancer 35, 58–90 (2011).
Mohan, A. et al. High prevalence of malnutrition and deranged relationship between energy demands and food intake in advanced non-small cell lung cancer. Eur. J. Cancer Care https://doi.org/10.1111/ecc.12503 (2017).
Ohnuma, T. in Holland-Frei Cancer Medicine (BC Decker, 2003).
Marceca, G. P., Londhe, P. & Calore, F. Management of cancer cachexia: attempting to develop new pharmacological agents for new effective therapeutic options. Front. Oncol. 10, 298 (2020).
Prado, B. L. & Qian, Y. Anti-cytokines in the treatment of cancer cachexia. Ann. Palliat. Med. 8, 67–79 (2018).
Groarke, J. D. et al. Ponsegromab for the treatment of cancer cachexia. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2409515 (2024). This paper highlights the clinical implications of GDF-15 as a biomarker for cancer-associated cachexia and demonstrates that the inhibition of GDF-15 with ponsegromab increases weight gain and reduces cachexia symptoms.
Yang, L., Shao, Y., Gao, T., Bajinka, O. & Yuan, X. Current advances in cancer energy metabolism under dietary restriction: a mini review. Med. Oncol. 41, 209 (2024).
Cormie, P., Zopf, E. M., Zhang, X. & Schmitz, K. H. The impact of exercise on cancer mortality, recurrence, and treatment-related adverse effects. Epidemiol. Rev. 39, 71–92 (2017).
Henriquez-Olguin, C. et al. Cytosolic ROS production by NADPH oxidase 2 regulates muscle glucose uptake during exercise. Nat. Commun. 10, 4623 (2019).
Morville, T., Sahl, R. E., Moritz, T., Helge, J. W. & Clemmensen, C. Plasma metabolome profiling of resistance exercise and endurance exercise in humans. Cell Rep. 33, 108554 (2020).
Matsui, T., Soya, M. & Soya, H. Endurance and brain glycogen: a clue toward understanding central fatigue. Adv. Neurobiol. 23, 331–346 (2019).
Pagnotti, G. M. et al. Combating osteoporosis and obesity with exercise: leveraging cell mechanosensitivity. Nat. Rev. Endocrinol. 15, 339–355 (2019).
Shao, M. et al. Advances in the research on myokine-driven regulation of bone metabolism. Heliyon 10, e22547 (2024).
Hensley, C. T. et al. Metabolic heterogeneity in human lung tumors. Cell 164, 681–694 (2016).
Pisarsky, L. et al. Targeting metabolic symbiosis to overcome resistance to anti-angiogenic therapy. Cell Rep. 15, 1161–1174 (2016).
Jimenez-Valerio, G. et al. Resistance to antiangiogenic therapies by metabolic symbiosis in renal cell carcinoma PDX models and patients. Cell Rep. 15, 1134–1143 (2016).
Allen, E. et al. Metabolic symbiosis enables adaptive resistance to anti-angiogenic therapy that is dependent on mTOR Signaling. Cell Rep. 15, 1144–1160 (2016).
Warburg, O., Wind, F. & Negelein, E. The metabolism of tumors in the body. J. Gen. Physiol. 8, 519–530 (1927).
Cori, C. F. & Cori, G. T. The carbohydrate metabolism of tumors: II. Changes in the sugar, lactic acid, and CO2-combining power of blood passing through a tumor. J. Biochem. Chem. 65, 397–405 (1925).
Warburg, O. On the origin of cancer cells. Science 123, 309–314 (1956).
Liberti, M. V. & Locasale, J. W. The Warburg effect: how does it benefit cancer cells? Trends Biochem. Sci. 41, 211–218 (2016).
Liu, Y. et al. An overview: the diversified role of mitochondria in cancer metabolism. Int. J. Biol. Sci. 19, 897–915 (2023).
Wang, S. F., Tseng, L. M. & Lee, H. C. Role of mitochondrial alterations in human cancer progression and cancer immunity. J. Biomed. Sci. 30, 61 (2023).
Fendt, S. M. 100 years of the Warburg effect: a cancer metabolism endeavor. Cell 187, 3824–3828 (2024).
Demicco, M., Liu, X. Z., Leithner, K. & Fendt, S. M. Metabolic heterogeneity in cancer. Nat. Metab. 6, 18–38 (2024).
Carr, A. C. & Maggini, S. Vitamin C and immune function. Nutrients https://doi.org/10.3390/nu9111211 (2017).
Yoshii, K., Hosomi, K., Sawane, K. & Kunisawa, J. Metabolism of dietary and microbial vitamin B family in the regulation of host immunity. Front. Nutr. 6, 48 (2019).
Ni, S., Yuan, Y., Kuang, Y. & Li, X. Iron metabolism and immune regulation. Front. Immunol. 13, 816282 (2022).
Razaghi, A., Poorebrahim, M., Sarhan, D. & Björnstedt, M. Selenium stimulates the antitumour immunity: insights to future research. Eur. J. Cancer 155, 256–267 (2021).
Mora, J. R., Iwata, M. & von Andrian, U. H. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat. Rev. Immunol. 8, 685–698 (2008).
Shankar, A. H. & Prasad, A. S. Zinc and immune function: the biological basis of altered resistance to infection. Am. J. Clin. Nutr. 68, 447s–463s (1998).
Böttger, F., Vallés-Martí, A., Cahn, L. & Jimenez, C. R. High-dose intravenous vitamin C, a promising multi-targeting agent in the treatment of cancer. J. Exp. Clin. Cancer Res. 40, 343 (2021).
Schoenfeld, J. D. et al. O2⋅− and H2O2-mediated disruption of Fe metabolism causes the differential susceptibility of NSCLC and GBM cancer cells to pharmacological ascorbate. Cancer Cell 31, 487–500.e8 (2017).
Ou, J. et al. The safety and pharmacokinetics of high dose intravenous ascorbic acid synergy with modulated electrohyperthermia in Chinese patients with stage III-IV non-small cell lung cancer. Eur. J. Pharm. Sci. 109, 412–418 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/show/NCT02420314 (2024).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/show/NCT02655913 (2018).
Ma, Y. et al. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci. Transl. Med. 6, 222ra218 (2014).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/show/NCT00228319 (2018).
Rothwell, P. M. et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet 379, 1602–1612 (2012).
Melhem-Bertrandt, A. et al. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J. Clin. Oncol. 29, 2645–2652 (2011).
Singhal, S. et al. Antitumor activity of thalidomide in refractory multiple myeloma. N. Engl. J. Med. 341, 1565–1571 (1999).
Barnard, R. A. et al. Autophagy inhibition delays early but not late-stage metastatic disease. J. Pharmacol. Exp. Ther. 358, 282–293 (2016).
Zeh, H. J. et al. A randomized phase II preoperative study of autophagy inhibition with high-dose hydroxychloroquine and gemcitabine/Nab-paclitaxel in pancreatic cancer patients. Clin. Cancer Res. 26, 3126–3134 (2020).
Pollak, M. Metformin and other biguanides in oncology: advancing the research agenda. Cancer Prev. Res. 3, 1060–1065 (2010).
Bosetti, C. et al. Cancer risk for patients using thiazolidinediones for type 2 diabetes: a meta-analysis. Oncologist 18, 148–156 (2013).
Meloni, A. R., DeYoung, M. B., Lowe, C. & Parkes, D. G. GLP-1 receptor activated insulin secretion from pancreatic β-cells: mechanism and glucose dependence. Diabetes Obes. Metab. 15, 15–27 (2013).
Carlessi, R. et al. GLP-1 receptor signalling promotes β-cell glucose metabolism via mTOR-dependent HIF-1α activation. Sci. Rep. 7, 2661 (2017).
Raven, L. M., Stoita, A., Feller, R. B., Brown, C. & Greenfield, J. R. Delayed gastric emptying with perioperative use of glucagon-like peptide-1 receptor agonists. Am. J. Med. 136, e233–e234 (2023).
Nakatani, Y. et al. Effect of GLP-1 receptor agonist on gastrointestinal tract motility and residue rates as evaluated by capsule endoscopy. Diabetes Metab. 43, 430–437 (2017).
Shah, M. & Vella, A. Effects of GLP-1 on appetite and weight. Rev. Endocr. Metab. Disord. 15, 181–187 (2014).
Wang, L., Wang, W., Kaelber, D. C., Xu, R. & Berger, N. A. GLP-1 receptor agonists and colorectal cancer risk in drug-naive patients with type 2 diabetes, with and without overweight/obesity. JAMA Oncol. 10, 256–258 (2024). This paper shows that GLP-1 receptor agonists can be used for weight loss, as their usage has exploded in popularity in recent years,but there is undoubtedly a wealth of information still to be discovered about their potential role in other diseases such as cancer.
De Barra, C. et al. Glucagon-like peptide-1 therapy in people with obesity restores natural killer cell metabolism and effector function. Obesity 31, 1787–1797 (2023).
Wolf, N. K., Kissiov, D. U. & Raulet, D. H. Roles of natural killer cells in immunity to cancer, and applications to immunotherapy. Nat. Rev. Immunol. 23, 90–105 (2023).
Michelet, X. et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat. Immunol. 19, 1330–1340 (2018).
O’Brien, K. L. & Finlay, D. K. Immunometabolism and natural killer cell responses. Nat. Rev. Immunol. 19, 282–290 (2019).
Acknowledgements
P.A.-M. is supported by the European Research Council Starting Grant (101116912) and the Ramón y Cajal Program. A.D.-F. is funded by Cancer Center Scholar T32 Grant (20910820). T.J. is supported by research grants from the National Institutes of Health (1R37CA286477-01A1, 1OT2CA278690-03), the Cancer Research Institute (CGCATF-2021/100019), the Mark Foundation (20-028-EDV), the STARR Cancer Consortium (I15-0037), and the Simons Foundation (1142664). A.E. is supported by research grants from Minerva, Israel Ministry of Health, the European Research Council (PoC 101111915), the Israel Science Foundation (873/23), and The Israel Cancer Research Fund (837124). A.E. is the incumbent of the Sir Ernst B. Chain Professorial Chair. The Moross Integrated Cancer Center, EKARD Institute for Cancer Diagnosis Research, Abisch-Frenkel RNA Therapeutics Center, G. S. Omenn and M. A. Darling, and the Koret Foundation generously supported the research performed by A.E.’s laboratory. Grammarly and ChatGPT were used for English editing.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
T.J. received consultancy fees from LepTx and Flagship Pioneering, not related to this manuscript. A.E. shares patent rights with the startup companies OnVagus and MetaboCure. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cancer thanks Navdeep Chandel, Andrew Hoy and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Agouti-related peptide (AgRP) neurons
-
Specialized neurons within the hypothalamus that modulate food intake and energy expenditure through recognition of AgRP levels.
- Cachexia
-
A complex metabolic syndrome often associated with end-stage cancer that is characterized by severe weight loss, muscle atrophy, fatigue, weakness and loss of appetite, and the condition is not reversible by nutritional support alone.
- Caloric restriction
-
A dietary regimen that reduces calorie intake without incurring malnutrition, which has been associated with lifespan extension and reduced incidence of age-related diseases in some animal models.
- Fatty acid activation
-
The process by which fatty acids are converted into fatty acyl-CoA molecules, making them metabolically active and ready for subsequent reactions.
- Ferroptosis
-
Iron-dependent form of regulated cell death caused by excessive accumulation of ROS and oxidative damage to membrane lipids, leading to loss of membrane integrity.
- Glucagon-like peptide-1
-
(GLP-1). A hormone primarily produced in the intestine, pancreas and brain that is implicated in insulin production, glucagon suppression, gastric emptying and satiety.
- Ketogenic diet
-
A high-fat, low-carbohydrate diet that induces a metabolic state known as ketosis, wherein the body relies on ketone bodies for energy instead of glucose.
- Ketone bodies
-
Water-soluble molecules (acetoacetate, β-hydroxybutyrate and acetone) produced from fatty acids in the liver that can serve as an alternative energy source, particularly for the brain, during low food intake, carbohydrate-restricted diets, starvation or prolonged intense exercise.
- Lipid peroxidation
-
A process in which free radicals steal electrons from the lipids in cell membranes, resulting in cell damage. This oxidative degradation of lipids is a key mechanism of cell injury and is implicated in various diseases, including cancer.
- Liver zonation
-
The spatial heterogeneity of metabolic processes in the liver, wherein different zones (periportal, midzonal and perivenous) perform distinct metabolic functions to efficiently regulate various physiological processes, such as detoxification, glucose production, and ammonia metabolism.
- Metabolic dysfunction-associated steatotic liver disease
-
(MASLD). A liver disease previously known as non-alcoholic fatty liver disease that is associated with metabolic dysfunction and characterized by excess fat accumulation in the liver.
- Metabolic symbiosis
-
A cooperative interaction between different types of cells within a tumour and its microenvironment, wherein they exchange metabolites to support cancer growth and survival under conditions of metabolic stress, such as hypoxia or nutrient scarcity.
- Metabolic syndrome
-
A cluster of conditions — including increased blood pressure, high blood sugar levels, excess body fat around the waist, and abnormal cholesterol levels — that occur together, increasing the risk of heart disease, stroke and type 2 diabetes.
- Metastatic tropism
-
The tendency of cancer cells to preferentially metastasize to specific organs, which is influenced by factors such as the organ metabolic environment, the expression of specific receptors on cancer cells, and the production of chemokines by target organs.
- Pro-opiomelanocortin (POMC) neurons
-
Specialized neurons within the hypothalamus that monitor global energy balance and control appetite through recognition of POMC levels.
- Reactive oxygen species
-
(ROS). Chemically reactive molecules containing oxygen, such as peroxides and superoxides, that have roles in cell signalling and homeostasis and, if excessively produced, can lead to oxidative stress and cellular damage, thereby contributing to ageing and diseases such as cancer.
- Sarcopenia
-
The progressive loss of muscle mass and strength owing to normal ageing.
- Senescent cells
-
Cells that have irreversibly stopped dividing and entered a metabolically active survival state in response to cellular stress or as a consequence of ageing.
- Tumour macroenvironment
-
The broader physiological environment in which a tumour exists, extending beyond the immediate tumour microenvironment, encompassing the surrounding tissues, organs, immune system and systemic factors in the body, such as hormones, cytokines and nutrients, that can influence tumour growth and progression.
- Tumour microenvironment
-
(TME). The complex ecosystem surrounding and influencing a tumour, which includes cellular and non-cellular components, such as fibroblasts, immune cells, endothelia, nutrients, extracellular matrix, and nutrients.
- Warburg effect
-
A metabolic shift that supports rapid cell growth and proliferation in which cancer cells predominantly produce energy by anaerobic glycolysis rather than oxidative phosphorylation, even when oxygen is abundant, leading to lactic acid fermentation in the cytosol.
- Western diet
-
Dietary pattern characterized by high intakes of saturated fats, refined sugars, processed foods and low consumption of fibre, fruits, vegetables and whole grains, which is often linked to nutrient imbalances.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Altea-Manzano, P., Decker-Farrell, A., Janowitz, T. et al. Metabolic interplays between the tumour and the host shape the tumour macroenvironment. Nat Rev Cancer (2025). https://doi.org/10.1038/s41568-024-00786-4
Accepted:
Published:
DOI: https://doi.org/10.1038/s41568-024-00786-4