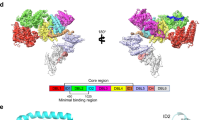
Abstract
Plasmodium falciparum VAR2CSA binds to chondroitin sulfate A (CSA) on the surface of the syncytiotrophoblast during placental malaria. This interaction facilitates placental sequestration of malaria parasites resulting in severe health outcomes for both the mother and her offspring. Furthermore, CSA is presented by diverse cancer cells and specific targeting of cells by VAR2CSA may become a viable approach for cancer treatment. In the present study, we determined the cryo-electron microscopy structures of the full-length ectodomain of VAR2CSA from P. falciparum strain NF54 in complex with CSA, and VAR2CSA from a second P. falciparum strain FCR3. The architecture of VAR2CSA is composed of a stable core flanked by a flexible arm. CSA traverses the core domain by binding within two channels and CSA binding does not induce major conformational changes in VAR2CSA. The CSA-binding elements are conserved across VAR2CSA variants and are flanked by polymorphic segments, suggesting immune selection outside the CSA-binding sites. This work provides paths for developing interventions against placental malaria and cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request. Atomic coordinates have been deposited at the Protein Data Bank under accession nos. PDB 7JGD (VAR2CSA core crosslink), PDB 7JGE (VAR2CSA FCR3 core), PDB 7JGF (VAR2CSA FCR3 DBL5 and DBL6), PDB 7JGG (VAR2CSA NF54 DBL5 and DBL6) and PDB 7JGH (VAR2CSA NF54 + CSA core), and cryo-EM density maps have been deposited at the Electron Microscopy Data Bank (EMDB) under accession nos. EMD 22323 (VAR2CSA core crosslink), EMD 22324 (VAR2CSA FCR3), EMD 22325 (VAR2CSA FCR3 DBL5 and DBL6), EMD 22326 (VAR2CSA NF54 DBL5 and DBL6) and EMD 22327 (VAR2CSA NF54 core + CSA). Source data are provided with this paper.
References
Fried, M. & Duffy, P. E. Malaria during pregnancy. Cold Spring Harb. Perspect. Med. 7, https://doi.org/10.1101/cshperspect.a025551 (2017).
Desai, M. et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect. Dis. 7, 93–104 (2007).
Zakama, A. K., Ozarslan, N. & Gaw, S. L. Placental malaria. Curr. Trop. Med. Rep. https://doi.org/10.1007/s40475-020-00213-2 (2020).
Guyatt, H. L. & Snow, R. W. The epidemiology and burden of Plasmodium falciparum-related anemia among pregnant women in sub-Saharan Africa. Am. J. Trop. Med. Hyg. 64, 36–44 (2001).
Steketee, R. W., Nahlen, B. L., Parise, M. E. & Menendez, C. The burden of malaria in pregnancy in malaria-endemic areas. Am. J. Trop. Med. Hyg. 64, 28–35 (2001).
Duffy, P. E. & Fried, M. Plasmodium falciparum adhesion in the placenta. Curr. Opin. Microbiol. 6, 371–376 (2003).
Ndam, N. T. et al. Protective antibodies against placental malaria and poor outcomes during pregnancy, Benin. Emerg. Infect. Dis. 21, 813–823 (2015).
Pasternak, N. D. & Dzikowski, R. PfEMP1: an antigen that plays a key role in the pathogenicity and immune evasion of the malaria parasite Plasmodium falciparum. Int. J. Biochem. Cell B 41, 1463–1466 (2009).
Salanti, A. et al. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J. Exp. Med. 200, 1197–1203 (2004).
Clausen, T. M. et al. Structural and functional insight into how the Plasmodium falciparum VAR2CSA protein mediates binding to chondroitin sulfate A in placental malaria. J. Biol. Chem. 287, 23332–23345 (2012).
Fried, M. & Duffy, P. E. Designing a VAR2CSA-based vaccine to prevent placental malaria. Vaccine 33, 7483–7488 (2015).
Trimnell, A. R. et al. Global genetic diversity and evolution of var genes associated with placental and severe childhood malaria. Mol. Biochem. Parasitol. 148, 169–180 (2006).
Bockhorst, J. et al. Structural polymorphism and diversifying selection on the pregnancy malaria vaccine candidate VAR2CSA. Mol. Biochem. Parasitol. 155, 103–112 (2007).
Srivastava, A. et al. Full-length extracellular region of the var2CSA variant of PfEMP1 is required for specific, high-affinity binding to CSA. Proc. Natl Acad. Sci. USA 107, 4884–4889 (2010).
Bewley, M. C. et al. Molecular architecture and domain arrangement of the placental malaria protein VAR2CSA suggests a model for carbohydrate binding. J. Biol. Chem. https://doi.org/10.1074/jbc.RA120.014676 (2020).
Dahlback, M. et al. The chondroitin sulfate A-binding site of the VAR2CSA protein involves multiple N-terminal domains. J. Biol. Chem. 286, 15908–15917 (2011).
Srivastava, A. et al. Var2CSA mnimal CSA binding region is located within the N-terminal region. PLoS ONE 6, https://doi.org/10.1371/journal.pone.0020270 (2011).
Resende, M. et al. Chondroitin sulphate A (CSA)-binding of single recombinant Duffy-binding-like domains is not restricted to Plasmodium falciparum erythrocyte membrane protein 1 expressed by CSA-binding parasites. Int. J. Parasitol. 39, 1195–1204 (2009).
Khunrae, P., Philip, J. M. D., Bull, D. R. & Higgins, M. K. Structural comparison of two CSPG-binding DBL domains from the VAR2CSA protein important in malaria during pregnancy. J. Mol. Biol. 393, 202–213 (2009).
Babakhanyan, A. et al. The antibody response of pregnant Cameroonian women to VAR2CSA ID1-ID2a, a small recombinant protein containing the CSA-binding site. PLoS ONE 9, e88173 (2014).
Singh, K. et al. Structure of the DBL3x domain of pregnancy-associated malaria protein VAR2CSA complexed with chondroitin sulfate A. Nat. Struct. Mol. Biol. 15, 932–938 (2008).
Higgins, M. K. The structure of a chondroitin sulfate-binding domain important in placental malaria. J. Biol. Chem. 283, 21842–21846 (2008).
Chene, A. et al. Down-selection of the VAR2CSA DBL1-2 expressed in E. coli as a lead antigen for placental malaria vaccine development. NPJ Vaccines 3, 28 (2018).
Chene, A. et al. Clinical development of placental malaria vaccines and immunoassays harmonization: a workshop report. Malar. J. 15, 476 (2016).
Sirima, S. B. et al. PRIMVAC vaccine adjuvanted with Alhydrogel or GLA-SE to prevent placental malaria: a first-in-human, randomised, double-blind, placebo-controlled study. Lancet Infect. Dis. https://doi.org/10.1016/S1473-3099(19)30739-X (2020).
Mordmuller, B. et al. First-in-human, randomized, double-blind clinical trial of differentially adjuvanted PAMVAC, a vaccine candidate to prevent pregnancy-associated malaria. Clin. Infect. Dis. 69, 1509–1516 (2019).
Salanti, A. et al. Targeting human cancer by a glycosaminoglycan binding malaria protein. Cancer Cell 28, 500–514 (2015).
Agerbaek, M. O., Bang-Christensen, S. & Salanti, A. Fighting cancer using an oncofetal glycosaminoglycan-binding protein from malaria parasites. Trends Parasitol. 35, 178–181 (2019).
Agerbaek, M. O. et al. The VAR2CSA malaria protein efficiently retrieves circulating tumor cells in an EpCAM-independent manner. Nat. Commun. 9, https://doi.org/10.1038/s41467-018-05793-2 (2018).
Nordor, A. V., Bellet, D. & Siwo, G. H. Cancer–malaria: hidden connections. Open Biol. 8, https://doi.org/10.1098/rsob.180127 (2018).
Tolia, N. H., Enemark, E. J., Sim, B. K. & Joshua-Tor, L. Structural basis for the EBA-175 erythrocyte invasion pathway of the malaria parasite Plasmodium falciparum. Cell 122, 183–193 (2005).
Salinas, N. D., Tang, W. K. & Tolia, N. H. Blood-stage malaria parasite antigens: structure, function, and vaccine potential. J. Mol. Biol. https://doi.org/10.1016/j.jmb.2019.05.018 (2019).
Sim, B. K., Chitnis, C. E., Wasniowska, K., Hadley, T. J. & Miller, L. H. Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science 264, 1941–1944 (1994).
Adams, J. H. et al. A family of erythrocyte binding proteins of malaria parasites. Proc. Natl Acad. Sci. USA 89, 7085–7089 (1992).
Batchelor, J. D. et al. Red blood cell invasion by Plasmodium vivax: structural basis for DBP engagement of DARC. PLoS Pathog. 10, e1003869 (2014).
Lennartz, F., Smith, C., Craig, A. G. & Higgins, M. K. Structural insights into diverse modes of ICAM-1 binding by Plasmodium falciparum-infected erythrocytes. Proc. Natl Acad. Sci. USA 116, 20124–20134 (2019).
Lennartz, F. et al. Structure-guided identification of a family of dual receptor-binding PfEMP1 that is associated with cerebral malaria. Cell Host Microbe 21, 403–414 (2017).
Sander, A. F. et al. Multiple var2csa-type PfEMP1 genes located at different chromosomal loci occur in many Plasmodium falciparum isolates. PLoS ONE 4, https://doi.org/10.1371/journal.pone.0006667 (2009).
Holm, L. DALI and the persistence of protein shape. Protein Sci. 29, 128–140 (2020).
Vigan-Womas, I. et al. Structural basis for the ABO blood-group fependence of Plasmodium falciparum rosetting. PLoS Pathog. 8, https://doi.org/10.1371/journal.ppat.1002781 (2012).
Malpede, B. M., Lin, D. H. & Tolia, N. H. Molecular basis for sialic acid-dependent receptor recognition by the Plasmodium falciparum invasion protein erythrocyte-binding antigen-140/BAEBL. J. Biol. Chem. 288, 12406–12415 (2013).
Achur, R. N., Valiyaveettil, M. & Gowda, D. C. The low sulfated chondroitin sulfate proteoglycans of human placenta have sulfate group-clustered domains that can efficiently bind Plasmodium falciparum-infected erythrocytes. J. Biol. Chem. 278, 11705–11713 (2003).
Achur, R. N., Valiyaveettil, M., Alkhalil, A., Ockenhouse, C. F. & Gowda, D. C. Characterization of proteoglycans of human placenta and identification of unique chondroitin sulfate proteoglycans of the intervillous spaces that mediate the adherence of Plasmodium falciparum-infected erythrocytes to the placenta. J. Biol. Chem. 275, 40344–40356 (2000).
Agbor-Enoh, S. T. et al. Chondroitin sulfate proteoglycan expression and binding of Plasmodium falciparum-infected erythrocytes in the human placenta during pregnancy. Infect. Immun. 71, 2455–2461 (2003).
Alkhalil, A., Achur, R. N., Valiyaveettil, M., Ockenhouse, C. F. & Gowda, D. C. Structural requirements for the adherence of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate proteoglycans of human placenta. J. Biol. Chem. 275, 40357–40364 (2000).
Gangnard, S. et al. Structure of the DBL3X–DBL4 epsilon region of the VAR2CSA placental malaria vaccine candidate: insight into DBL domain interactions. Sci. Rep. 5, https://doi.org/10.1038/srep14868 (2015).
Gangnard, S. et al. Structural and immunological correlations between the variable blocks of the VAR2CSA domain DBL6 epsilon from two Plasmodium falciparum parasite lines. J. Mol. Biol. 425, 1697–1711 (2013).
Avril, M. et al. Evidence for globally shared, cross-reacting polymorphic epitopes in the pregnancy-associated malaria vaccine candidate VAR2CSA. Infect. Immun. 76, 1791–1800 (2008).
Glaser, F. et al. ConSurf: identification of functional regions in proteins by surface-mapping of phylogenetic information. Bioinformatics 19, 163–164 (2003).
Ditlev, S. B. et al. Identification and characterization of B-cell epitopes in the DBL4 epsilon domain of VAR2CSA. PLoS ONE 7, https://doi.org/10.1371/journal.pone.0043663 (2012).
Doritchamou, J. Y. A. et al. VAR2CSA domain-specific analysis of naturally acquired functional antibodies to Plasmodium falciparum placental malaria. J. Infect. Dis. 214, 577–586 (2016).
Barfod, L. et al. Human pregnancy-associated malaria-specific B cells target polymorphic, conformational epitopes in VAR2CSA. Mol. Microbiol. 63, 335–347 (2007).
Andersen, P. et al. Structural insight into epitopes in the pregnancy-associated malaria protein VAR2CSA. PLoS Pathog. 4, https://doi.org/10.1371/journal.ppat.0040042 (2008).
Mitran, C. J. et al. Antibodies to cryptic epitopes in distant homologues underpin a mechanism of heterologous immunity between Plasmodium vivax PvDBP and Plasmodium falciparum VAR2CSA. mBio 10, https://doi.org/10.1128/mBio.02343-19 (2019).
Peters, P. J., Thigpen, M. C., Parise, M. E. & Newman, R. D. Safety and toxicity of sulfadoxine/pyrimethamine: implications for malaria prevention in pregnancy using intermittent preventive treatment. Drug Saf. 30, 481–501 (2007).
Patel, J. C. et al. Increased risk of low birth weight in women with placental malaria associated with P. falciparum VAR2CSA clade. Sci. Rep. 7, 7768 (2017).
Doritchamou, J. Y. A. et al. Placental malaria vaccine candidate antigen VAR2CSA displays atypical domain architecture in some Plasmodium falciparum strains. Commun. Biol. 2, https://doi.org/10.1038/s42003-019-0704-z (2019).
Helms, G., Dasanna, A. K., Schwarz, U. S. & Lanzer, M. Modeling cytoadhesion of Plasmodium falciparum-infected erythrocytes and leukocytes—common principles and distinctive features. FEBS Lett. 590, 1955–1971 (2016).
Cutts, E. E. et al. Structural analysis of P. falciparum KAHRP and PfEMP1 complexes with host erythrocyte spectrin suggests a model for cytoadherent knob protrusions. PLoS Pathog. 13, https://doi.org/10.1371/journal.ppat.1006552 (2017).
Dorin-Semblat, D. et al. Phosphorylation of the VAR2CSA extracellular region is associated with enhanced adhesive properties to the placental receptor CSA. PLoS Biol. 17, https://doi.org/10.1371/journal.pbio.3000308 (2019).
Doritchamou, J. et al. Differential adhesion-inhibitory patterns of antibodies raised against two major variants of the NTS-DBL2X region of VAR2CSA. Vaccine 31, 4516–4522 (2013).
Akhouri, R. R., Goel, S., Furusho, H., Skoglund, U. & Wahlgren, M. Architecture of human IgM in complex with P. falciparum erythrocyte membrane protein 1. Cell Rep. 14, 723–736 (2016).
Brown, A. et al. Molecular architecture of a complex between an adhesion protein from the malaria parasite and intracellular adhesion molecule 1. J. Biol. Chem. 288, 5992–6003 (2013).
Stevenson, L. et al. Investigating the function of Fc-specific binding of IgM to Plasmodium falciparum erythrocyte membrane protein 1 mediating erythrocyte rosetting. Cell Microbiol. 17, 819–831 (2015).
Wahlgren, M., Goel, S. & Akhouri, R. R. Variant surface antigens of Plasmodium falciparum and their roles in severe malaria. Nat. Rev. Microbiol. 15, 479–491 (2017).
Shukla, A. K. et al. Visualization of arrestin recruitment by a G-protein-coupled receptor. Nature 512, 218–222 (2014).
Ohi, M., Li, Y., Cheng, Y. & Walz, T. Negative staining and image cassification—powerful tools in modern electron microscopy. Biol. Proced. Online 6, 23–34 (2004).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Suloway, C. et al. Automated molecular microscopy: the new Leginon system. J. Struct. Biol. 151, 41–60 (2005).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Zhang, K. Gctf: real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Afonine, P. V. et al. New tools for the analysis and validation of cryo-EM maps and atomic models. Acta Crystallogr. D Struct. Biol. 74, 814–840 (2018).
Kelley, L. A., Mezulis, S., Yates, C. M., Wass, M. N. & Sternberg, M. J. E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 10, 845–858 (2015).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D Biol. Crystallogr. 58, 1948–1954 (2002).
Pettersen, E. F. et al. UCSF chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Whalen, D. M., Malinauskas, T., Gilbert, R. J. C. & Siebold, C. Structural insights into proteoglycan-shaped Hedgehog signaling. Proc. Natl Acad. Sci. USA 110, 16420–16425 (2013).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Acknowledgements
This work was funded by the Intramural Research Programs of the National Institute of Allergy and Infectious Diseases (NIAID) and the National Institute of Child Health and Human Development, NIH. We thank NIH MICEF and NCI/NICE for the data collection. We thank J. Jiang for instrument access and expertise on sample preparation and data processing. We thank E. Fischer, V. Nair, C. Schwartz, B. Hansen, A. Dearborn and J. Marcotrigiano for their assistance in initial sample preparation and analysis. We thank N. Salinas, T. Dickey and W. Tang for their constructive suggestions on the manuscript. We thank J. Patrick Gorres for assistance in editing the manuscript. The present study used the Office of Cyber Infrastructure and Computational Biology High Performance Computing cluster at the NIAID, Bethesda, MD.
Author information
Authors and Affiliations
Contributions
N.H.T. conceived the study, and designed and supervised research. R.M. designed and carried out all the experiments and data analysis. J.P.R., with support from P.E.D., cloned the original expression plasmids to generate a panel of full-length VAR2CSA variants. T.L. assisted with grid freezing, cryo-EM data collection and data processing of crosslinked VAR2CSA FCR3. R.H. assisted with cryo-EM data collection of VAR2CSA FCR3 and the VAR2CSA NF54 CSA complex. J.D.P. and J.Z. helped with the negative-stain studies. R.M. and N.H.T. interpreted the data and wrote the manuscript, with input from all the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Microbiology thanks Lars Hviid, Stephanie Yanow and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 VAR2CSA Protein purification.
a, Domain boundaries of VAR2CSA NF54 and VAR2CSA FCR3 ectodomains we used in the structural analysis. b, Top: Size Exclusion Chromatography (SEC) profile of the VAR2CSA NF54 (orange) and VAR2CSA FCR3 proteins. Bottom: SDS PAGE analysis of the corresponding SEC fractions of VAR2CSA NF54 (left) and VAR2CSA FCR3 (right). Similar data were obtained from three independent purifications.
Extended Data Fig. 2 Data-processing pipeline for the cryo-EM movies of CSA-VAR2CSA NF54 complex.
a, Flow chart showing the image-processing pipeline for the cryo-EM data of VAR2CSA starting with 6,196 dose-fractionated movies collected on a 300-keV Titan Krios (FEI) equipped with a K2 Summit direct electron detector (Gatan). Data were processed in cryoSPARC. Full frame motion correction was done by cryoSPARC’s own implementation. CTF estimation for each micrograph was calculated with Gctf. Particles were autopicked from each micrograph with the blob picker from cryoSPARC and then sorted by 2D classification for two rounds. The twelve highest-populated classes with clear features from the 2D classification are shown. The dataset contained 858,299 particles. A subset of particles was used to generate an ab initio map in cryoSPARC. Particles were classified into 5 classes using the low-pass-filtered (30 Å) ab initio map as a template. Class 1 was selected with 157,702 particles to conduct NU-refinement and generated a 3.87 Å map. A mask covering DBL5ɛ and DBL6ɛ domains were then used to perform local refinement and generated a 4.88 Å map. Class 1 and Class 4 which have a clear core density were selected again with 299,571 particles to conduct NU-refinement and generated a 3.5 Å map, local refinement improved the resolution of the core to 3.36 Å. Scale bar: 10 nm. Analysis was performed three times independently with similar results. b, Gold-standard FSC curves are shown. The dotted line represents the 0.143 FSC cut-off. c, Angular distribution calculated in cryoSPARC for particle projections of the full-length protein (right) and the core (left). Heat map shows number of particles for each viewing angle. d,e and f, Local resolution of the core (d), full length (e) and arm region (f) in two views. The representation of colors for different resolution are shown on the right. g, FSC calculated between the refined structures and the full map. h, Representative cryo-EM densities from the core machinery map. i, Representative cryo-EM densities from the arm with DBL5ɛ and DBL6ɛ model docked in.
Extended Data Fig. 3 Structural conservation with the PfEMP1 family.
a, Structural alignment of DBL1X with VarO_DBL1α1 (PDB: 2YK0, RMSD:3.18) and IT4var13 DBLβ (PDB:6s8t, RMSD: 2.94). b, Structural alignment of DBL2X with varO_DBL1α1 (PDB: 2YK0, RMSD:5.75) and PF11_0521_ DBLβ (PDB: 5mza, RMSD:4.85). c, Structural alignment of DBL5ɛ with IT4var13 DBLβ (PDB: 6s8t, RMSD:8.37) and EBA-175 F2 domain (PDB: 1ZRO, RMSD:4.24). d, DBL3X-4ɛ and e, DBL 5ɛ-6ɛ, DBL domains are colored according to Fig. 1a. f, Crystal structure of EBA-175 (PDB: 1ZRO). The F1 and F2 domain are colored in light and dark grey respectively. g, Crystal structure of EBA-140 (PDB: 4JNO). The F1 and F2 domain are colored in brown and maroon respectively. h, Structural comparison of VAR2CSA DBL2X-ID and PfEMP1-VarO DBL1α-CIDR. Upper: atomic model of VAR2CSA DBL2X-ID2; Lower: Crystal structure of varO_DBL1α1-CIDR γ. Green: DBL1α1, Gold: CIDR subdomain1, Grey: CIDR subdomain2. i, Structural alignment of VAR2CSA_ID2 with varO_CIDR γ subdomain2, RMSD = 4.43. j, Sequence alignment of VAR2CSA ID2 and varO_CIDR γ.
Extended Data Fig. 4 The recognition of CSA by VAR2CSA.
a, One ASG monosaccharide could be built in a weak density found in the minor binding channel sandwiched by DBL2X and ID2a. The density is shown in mesh. The ASG monosaccharide is colored in green. The residues that involve in forming hydrogen bonds with the ASG monosaccharide are illustrated. b, Electrostatic surface of the proteins showing both major binding channel and minor binding channel are positively charged. c, partial sequence alignment of the residues involved in the minor binding channel. The residues that interact with the monosaccharide from DBL2X and ID2a are highlighted on top by pink and blue spheres respectively.
Extended Data Fig. 5 Data-processing pipeline for the cryo-EM movies of apo VAR2CSA FCR3 and VAR2CSA FCR3_crosslink.
a, Flow chart showing the image-processing pipeline for the cryo-EM data of VAR2CSA starting with 100,108 dose-fractionated movies collected on a 300-keV Titan Krios (FEI) equipped with a K2 Summit direct electron detector (Gatan). Processing was done within cryoSPARC. Full frame motion correction was done by cryoSPARC’s own implementation. CTF estimation for each micrograph was calculated with Gctf. Particles were autopicked from each micrograph with the blob picker from cryoSPARC and then sorted by 2D classification for two rounds to exclude bad particles. The twelve highest-populated classes with clear features from the 2D classification are shown. The dataset contained 783,088 particles. A subset of particles was used to generate an ab initio map in cryoSPARC. Particles were classified into 10 classes using the low-pass-filtered (30 Å) ab initio map as a template. Class 4 with a total of 271,442 particles was selected to conduct NU-refinement and generated a 4 Å map. A mask covering the arm region were then used to perform local refinement and generated a 4.69 Å map. The angular distribution calculated in cryoSPARC for particle projections are shown in heat map which shows number of particles for each viewing angle. Scale bar: 10 nm. Analysis was performed three times independently with similar results. b, Gold-standard FSC curves. The dotted line represents the 0.143 FSC cut-off, which indicates a nominal resolution of 4 Å (black) and 4.69 Å (blue) for the full length protein and arm region respectively. c, Local resolution of the full length VAR2CSA map in two views. The representation of colors for different resolution are shown on the right. d, Local resolution of the arm map in two views. The representation of colors for different resolution are shown on the right. e, Flow chart showing the image-processing pipeline for the cryo-EM data of crosslinked VAR2CSA starting with 4,739 dose-fractionated movies collected on a 300-keV Titan Krios (FEI) equipped with a K2 Summit direct electron detector (Gatan). All processing was done within cryoSPARC. Full frame motion correction was done by cryoSPARC’s own implementation and a sample. CTF estimation for each micrograph was calculated with Gctf. Particles were autopicked from each micrograph with the blob picker from cryoSPARC and then sorted by 2D classification for two rounds to exclude bad particles. The twelve highest-populated classes with clear features from the 2D classification are shown. The dataset contained 505,409 particles. A subset of particles was used to generate an ab initio map in cryoSPARC. Particles were classified into 3 classes using the low-pass-filtered (30 Å) ab initio map as a template. Class 3 was selected to conduct NU-refinement and generated a 3.52 Å map. A mask covering the core was then used to perform local refinement and generated a 3.38 Å map. The angular distribution calculated in cryoSPARC for particle projections are shown in heat map which shows number of particles for each viewing angle. Scale bar: 10 nm. Analysis was performed three times independently with similar results. f, Gold-standard FSC curves. The dotted line represents the 0.143 FSC cut-off, which indicates a nominal resolution of 3.38 Å of the core. g, Local resolution of the crosslinked VAR2CSA FCR3 core map in two views. The representation of colors for different resolution are shown on the right.
Extended Data Fig. 6 Model building and validation for VAR2CSA FCR3.
a, Atomic model of the core of the crosslinked VAR2CSA FCR3. b, FSC calculated between the refined structure and the full map. c, Representative cryo-EM densities from the core. d, Atomic model of full length VAR2CSA FCR3 docked in the 4.06 Å map. e, FSC calculated between the refined core structure and the full map. f, Representative cryo-EM densities from the core. g, Representative cryo-EM densities from the arm with DBL5ɛ and DBL6ɛ model docked in.
Extended Data Fig. 7 The model for VAR2CSA mediated placental malaria and cancer therapy.
a, The mechanism of placental sequestration of P. falciparum. In the placenta, the parasite express VAR2CSA on to the surface of the infected erythrocytes. VAR2CSA specifically binds to the CSA on the placental syncytiotrophoblast through a major and a potential minor CSA binding channel in its core with high affinity, leading to the sequestration of the parasite in the placenta and threaten the health of both the mother and their baby. b, Cancer cells of many cancer types harbor the same type of CSA on their surface as placenta. Conjugated VAR2CSA can be used to deliver drugs or labels specifically to tumor cells for therapeutics or diagnostics.
Extended Data Fig. 8 Analysis of rVAR2 for cancer therapy.
The structural model of rVAR2 are shown in ribbon. The remainder of the VAR2CSA protein is shown in surface. Each individual DBL and ID domain is colored according to Fig. 1a.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2.
Supplementary Video 1
Three-dimensional variability analysis of VAR2CSA NF54 in complex with CSA.
Supplementary Video 2
Three-dimensional variability analysis of VAR2CSA FCR3.
Supplementary Video 3
Multiple CSA-binding channel within the core structure of VAR2CSA NF54.
Rights and permissions
About this article
Cite this article
Ma, R., Lian, T., Huang, R. et al. Structural basis for placental malaria mediated by Plasmodium falciparum VAR2CSA. Nat Microbiol 6, 380–391 (2021). https://doi.org/10.1038/s41564-020-00858-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-020-00858-9