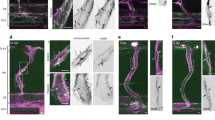
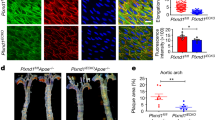
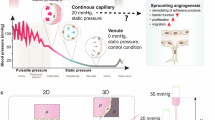
Abstract
Physiological blood flow induces the secretion of vasoactive compounds, notably nitric oxide, and promotes endothelial cell elongation and reorientation parallel to the direction of applied shear. How shear is sensed and relayed to intracellular effectors is incompletely understood. Here, we demonstrate that an apical spectrin network is essential to convey the force imposed by shear to endothelial mechanosensors. By anchoring CD44, spectrins modulate the cell surface density of hyaluronan and sense and translate shear into changes in plasma membrane tension. Spectrins also regulate the stability of apical caveolae, where the mechanosensitive PIEZO1 channels are thought to reside. Accordingly, shear-induced PIEZO1 activation and the associated calcium influx were absent in spectrin-deficient cells. As a result, cell realignment and flow-induced endothelial nitric oxide synthase stimulation were similarly dependent on spectrin. We conclude that the apical spectrin network is not only required for shear sensing but also transmits and distributes the resulting tensile forces to mechanosensors that elicit protective and vasoactive responses.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Source data are provided with this paper. All other data supporting the findings of this study are available from the corresponding author on reasonable request.
Code availability
The code generated during the current study are available from the corresponding author on reasonable request.
References
Davies, P. F. Flow-mediated endothelial mechanotransduction. Physiol. Rev. 75, 519–560 (1995).
Hahn, C. & Schwartz, M. A. Mechanotransduction in vascular physiology and atherogenesis. Nat. Rev. Mol. Cell Biol. 10, 53–62 (2009).
Radomski, M., Palmer, R. & Moncada, S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet 330, 1057–1058 (1987).
Radomski, M. W., Palmer, R. M. & Moncada, S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br. J. Pharmacol. 92, 639–646 (1987).
Dewey, C. F. Jr., Bussolari, S. R., Gimbrone, M. A. Jr. & Davies, P. F. The dynamic response of vascular endothelial cells to fluid shear stress. J. Biomech. Eng. 103, 177–185 (1981).
Barbee, K. A., Davies, P. F. & Lal, R. Shear stress-induced reorganization of the surface topography of living endothelial cells imaged by atomic force microscopy. Circ. Res. 74, 163–171 (1994).
Barbee, K. A., Mundel, T., Lal, R. & Davies, P. F. Subcellular distribution of shear stress at the surface of flow-aligned and nonaligned endothelial monolayers. Am. J. Physiol. 268, H1765–H1772 (1995).
Newman, P. J. The biology of PECAM-1. J. Clin. Invest. 99, 3–8 (1997).
Tzima, E. et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437, 426–431 (2005).
Conway, D. E. et al. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr. Biol. 23, 1024–1030 (2013).
Jin, Z.-G. et al. Ligand-independent activation of vascular endothelial growth factor receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthase. Circ. Res. 93, 354–363 (2003).
Duncan, G. S. et al. Genetic evidence for functional redundancy of platelet/endothelial cell adhesion molecule-1 (PECAM-1): CD31-deficient mice reveal PECAM-1-dependent and PECAM-1-independent functions. J. Immunol. 162, 3022–3030 (1999).
Aruffo, A., Stamenkovic, I., Melnick, M., Underhill, C. B. & Seed, B. CD44 is the principal cell surface receptor for hyaluronate. Cell 61, 1303–1313 (1990).
Mochizuki, S. et al. Role of hyaluronic acid glycosaminoglycans in shear-induced endothelium-derived nitric oxide release. Am. J. Physiol. Heart Circ. Physiol. 285, H722–H726 (2003).
Tarbell, J. M. & Ebong, E. E. The endothelial glycocalyx: a mechano-sensor and-transducer. Sci. Signal. 1, pt8 (2008).
Pahakis, M. Y., Kosky, J. R., Dull, R. O. & Tarbell, J. M. The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem. Biophys. Res. Commun. 355, 228–233 (2007).
Li, J. et al. Piezo1 integration of vascular architecture with physiological force. Nature 515, 279–282 (2014).
Mylvaganam, S. et al. Stabilization of endothelial receptor arrays by a polarized spectrin cytoskeleton facilitates rolling and adhesion of leukocytes. Cell Rep. 31, 107798 (2020).
Sheetz, M. P., Schindler, M. & Koppel, D. E. Lateral mobility of integral membrane proteins is increased in spherocytic erythrocytes. Nature 285, 510–512 (1980).
Xu, K., Zhong, G. & Zhuang, X. Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science 339, 452–456 (2013).
Bennett, V. & Healy, J. Membrane domains based on ankyrin and spectrin associated with cell–cell interactions. Cold Spring Harb. Perspect. Biol. 1, a003012 (2009).
Bennett, V. & Lorenzo, D. N. An adaptable spectrin/ankyrin-based mechanism for long-range organization of plasma membranes in vertebrate tissues. Curr. Top. Membr. 77, 143–184 (2016).
Doucette, J. W. et al. Validation of a Doppler guide wire for intravascular measurement of coronary artery flow velocity. Circulation 85, 1899–1911 (1992).
Shen, J., Luscinskas, F. W., Connolly, A., Dewey, C. F. Jr & Gimbrone, M. Jr Fluid shear stress modulates cytosolic free calcium in vascular endothelial cells. Am. J. Physiol. 262, C384–C390 (1992).
Buga, G. M., Gold, M. E., Fukuto, J. M. & Ignarro, L. J. Shear stress-induced release of nitric oxide from endothelial cells grown on beads. Hypertension 17, 187–193 (1991).
Lückhoff, A., Pohl, U., Mülsch, A. & Busse, R. Differential role of extra‐ and intracellular calcium in the release of EDRF and prostacyclin from cultured endothelial cells. Br. J. Pharmacol. 95, 189–196 (1988).
Uematsu, M. et al. Regulation of endothelial cell nitric oxide synthase mRNA expression by shear stress. Am. J. Physiol. 269, C1371–C1378 (1995).
Hong, D., Jaron, D., Buerk, D. G. & Barbee, K. A. Heterogeneous response of microvascular endothelial cells to shear stress. Am. J. Physiol. Heart Circ. Physiol. 290, H2498–H2508 (2006).
Weinbaum, S., Tarbell, J. M. & Damiano, E. R. The structure and function of the endothelial glycocalyx layer. Annu. Rev. Biomed. Eng. 9, 121–167 (2007).
Law, R. et al. Pathway shifts and thermal softening in temperature-coupled forced unfolding of spectrin domains. Biophys. J. 85, 3286–3293 (2003).
Lenne, P. F., Raae, A. J., Altmann, S. M., Saraste, M. & Horber, J. K. States and transitions during forced unfolding of a single spectrin repeat. FEBS Lett. 476, 124–128 (2000).
Rief, M., Pascual, J., Saraste, M. & Gaub, H. E. Single molecule force spectroscopy of spectrin repeats: low unfolding forces in helix bundles. J. Mol. Biol. 286, 553–561 (1999).
Law, R. et al. Cooperativity in forced unfolding of tandem spectrin repeats. Biophys. J. 84, 533–544 (2003).
Meng, F. & Sachs, F. Orientation-based FRET sensor for real-time imaging of cellular forces. J. Cell Sci. 125, 743–750 (2012).
Bennett, V. & Baines, A. J. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol. Rev. 81, 1353–1392 (2001).
Grum, V. L., Li, D., MacDonald, R. I. & Mondragón, A. Structures of two repeats of spectrin suggest models of flexibility. Cell 98, 523–535 (1999).
Sheetz, M. P. & Singer, S. On the mechanism of ATP-induced shape changes in human erythrocyte membranes. I. The role of the spectrin complex. J. Cell Biol. 73, 638–646 (1977).
Colom, A. et al. A fluorescent membrane tension probe. Nat. Chem. 10, 1118–1125 (2018).
Schwarz, G., Callewaert, G., Droogmans, G. & Nilius, B. Shear stress‐induced calcium transients in endothelial cells from human umbilical cord veins. J. Physiol. 458, 527–538 (1992).
Yamamoto, K., Korenaga, R., Kamiya, A. & Ando, J. Fluid shear stress activates Ca2+ influx into human endothelial cells via P2X4 purinoceptors. Circ. Res. 87, 385–391 (2000).
Wang, S. et al. P2Y2 and Gq/G11 control blood pressure by mediating endothelial mechanotransduction. J. Clin. Invest. 125, 3077–3086 (2015).
Bae, C., Sachs, F. & Gottlieb, P. A. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry 50, 6295–6300 (2011).
Syeda, R. et al. Piezo1 channels are inherently mechanosensitive. Cell Rep. 17, 1739–1746 (2016).
Diem, K. et al. Mechanical stretch activates Piezo1 in caveolae of alveolar type I cells to trigger ATP release and paracrine stimulation of surfactant secretion from alveolar type II cells. FASEB J. 34, 12785–12804 (2020).
Liang, X. & Howard, J. Structural biology: Piezo senses tension through curvature. Curr. Biol. 28, R357–R359 (2018).
Simionescu, N., Simionescu, M. & Palade, G. E. Differentiated microdomains on the luminal surface of the capillary endothelium. I. Preferential distribution of anionic sites. J. Cell Biol. 90, 605–613 (1981).
Parton, R. G. & Simons, K. The multiple faces of caveolae. Nat. Rev. Mol. Cell Biol. 8, 185–194 (2007).
Sinha, B. et al. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 144, 402–413 (2011).
Ridone, P. et al. Disruption of membrane cholesterol organization impairs the activity of PIEZO1 channel clusters. J. Gen. Physiol. 152, e201912515 (2020).
Parton, R. G. & del Pozo, M. A. Caveolae as plasma membrane sensors, protectors and organizers. Nat. Rev. Mol. Cell Biol. 14, 98–112 (2013).
Drab, M. et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 293, 2449–2452 (2001).
Oh, P. et al. Live dynamic imaging of caveolae pumping targeted antibody rapidly and specifically across endothelium in the lung. Nat. Biotechnol. 25, 327–337 (2007).
Michel, J. B., Feron, O., Sacks, D. & Michel, T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J. Biol. Chem. 272, 15583–15586 (1997).
Dimmeler, S. et al. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399, 601–605 (1999).
Fleming, I., Fisslthaler, B., Dimmeler, S., Kemp, B. E. & Busse, R. Phosphorylation of Thr495 regulates Ca2+/calmodulin-dependent endothelial nitric oxide synthase activity. Circ. Res. 88, E68–E75 (2001).
Calvert, J. W. et al. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK–eNOS-mediated signaling. Diabetes 57, 696–705 (2008).
Bir, S. C., Xiong, Y., Kevil, C. G. & Luo, J. Emerging role of PKA/eNOS pathway in therapeutic angiogenesis for ischaemic tissue diseases. Cardiovasc. Res. 95, 7–18 (2012).
Rademakers, T. et al. Endothelial beta-2 spectrin: a critical plaque stiffness dependent regulator of microvessel leakage in human atherosclerotic plaque. Atherosclerosis 275, e129 (2018).
Miyazaki, T. et al. m-Calpain induction in vascular endothelial cells on human and mouse atheromas and its roles in VE-cadherin disorganization and atherosclerosis. Circulation 124, 2522–2532 (2011).
Chien, S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am. J. Physiol. Heart Circ. Physiol. 292, H1209–H1224 (2007).
Palmer, A., Mason, T. G., Xu, J., Kuo, S. C. & Wirtz, D. Diffusing wave spectroscopy microrheology of actin filament networks. Biophys. J. 76, 1063–1071 (1999).
Stankewich, M. C. et al. Cell organization, growth, and neural and cardiac development require αII-spectrin. J. Cell Sci. 124, 3956–3966 (2011).
Voas, M. G. et al. αII-spectrin is essential for assembly of the nodes of Ranvier in myelinated axons. Curr. Biol. 17, 562–568 (2007).
Lambert, S. & Bennett, V. From anemia to cerebellar dysfunction: a review of the ankyrin gene family. Eur. J. Biochem. 211, 1–6 (1993).
Lux, S. E., JoHN, K. M. & Karnovsky, M. Irreversible deformation of the spectrin–actin lattice in irreversibly sickled cells. J. Clin. Invest. 58, 955–963 (1976).
Chasis, J., Agre, P. & Mohandas, N. Decreased membrane mechanical stability and in vivo loss of surface area reflect spectrin deficiencies in hereditary spherocytosis. J. Clin. Invest. 82, 617–623 (1988).
Agre, P., Orringer, E. P. & Bennett, V. Deficient red-cell spectrin in severe, recessively inherited spherocytosis. N. Engl. J. Med. 306, 1155–1161 (1982).
Guo, Y. R. & MacKinnon, R. Structure-based membrane dome mechanism for Piezo mechanosensitivity. eLife 6, e33660 (2017).
Lewis, A. H. & Grandl, J. Mechanical sensitivity of Piezo1 ion channels can be tuned by cellular membrane tension. eLife 4, e12088 (2015).
Hirama, T. et al. Phosphatidylserine dictates the assembly and dynamics of caveolae in the plasma membrane. J. Biol. Chem. 292, 14292–14307 (2017).
Chen, T.-W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Buchanan, C. F. et al. Three-dimensional microfluidic collagen hydrogels for investigating flow-mediated tumor-endothelial signaling and vascular organization. Tissue Eng. Part C Methods 20, 64–75 (2014).
Berezin, M. Y. & Achilefu, S. Fluorescence lifetime measurements and biological imaging. Chem. Rev. 110, 2641–2684 (2010).
Paulson, K. E. et al. Resident intimal dendritic cells accumulate lipid and contribute to the initiation of atherosclerosis. Circ. Res. 106, 383–390 (2010).
Freeman, S. A. et al. Transmembrane pickets connect cyto- and pericellular skeletons forming barriers to receptor engagement. Cell 172, 305–317.e10 (2018).
Acknowledgements
S.M. is supported by a Vanier Scholarship from the Canadian Institutes of Health Research (CIHR) and a SickKids Restracomp Studentship. S.A.F. and S.G. are supported by grants PJT-169180 and FDN-143202 from the CIHR.
Author information
Authors and Affiliations
Contributions
S.M. conducted experiments and data analyses. J.P. performed immunofluorescence experiments and cell-size measurements. B.Y. induced hypercholesterolaemia in mice and performed dissections. R.L. acquired electron micrographs and C.-Y.L. performed platelet isolations. L.A.R. provided reagents. S.M., S.A.F. and S.G. designed the study and wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cell Biology thanks Stephan Huveneers, Libin Liu, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Silencing of CD44 expression in endothelial cells.
a, CD44 expression in RF24 cells probed by immunoblotting after transfection with non-targeted or CD44-targeted siRNAs. Normalized to β-actin. Representative of 3 independent experiments. b, Representative confocal images of sparsely distributed endothelial cells immunostained for endogenous β-II spectrin (left panel and magenta in merge) and stained for actin filaments (F-actin) with phalloidin (green in merge). Representative of 3 independent experiments. c, Orthogonal confocal sections of cells immunostained for β-II spectrin following transfection with non-targeted or CD44 siRNA.
Extended Data Fig. 2 β-II spectrin-KO cells retain defining characteristics of the endothelium.
a, Distribution of VE-cadherin in indicated endothelial cell lines, assessed by immunofluorescence. Representative of 3 independent experiments. Here and elsewhere, scale bar: 5 μm. b, Ratio of VE-cadherin fluorescence at intercellular contacts and cytosol for individual cells in a confluent monolayer. In A and C jittered data points (in shades of blue) represent individual measurements and are color-coded according to biological replicate. Means of individual experiments are presented in black. Overall means ± SE are indicated in red. Histograms of all data points indicating the mean (solid line) as well as the 25th and 75th percentiles (dashed line) are shown to the right of the individual data. n = 3 independent experiments, each analyzing 23 cells per condition. c, Distribution of von-Willebrand factor (vWF) in indicated endothelial cell lines, assessed by immunofluorescence. n = 3, each analyzing 12 images per condition. d,e Distribution of vinculin (d) and p-tyrosine (e) at the basal cell surface in the indicated endothelial cell lines and F-actin following staining with phalloidin. n = 3 independent experiments of 12 images per condition. f, Mean fluorescence intensity (MFI) of phospho-tyrosine measured in confocal sections as in e, normalized to the experimental mean MFI of wildtype cells in static culture. Data are means ± SE, n = 3 independent experiments, each analyzing 12 images per condition. P values are from one-way ANOVA of experimental means (b,f).
Extended Data Fig. 3 β-II spectrin-KO cells fail to align with shear despite normal tyrosine phosphorylation of junctional proteins.
Endothelial cells were subjected to constant fluid flow or maintained in static culture for 30 min. Cells were then fixed and immunostained for phosphorylated protein tyrosine residues (p-tyrosine). a, Representative polar histograms of F-actin orientations analyzed as described in Methods from one representative experiment as in b. b, Confluent endothelial cells grown in static or shear conditions were fixed and immunostained for VE-cadherin to visualize cell borders. Aspect ratios were calculated as the longest axis divided by the shortest axis of each cell. Here and elsewhere, for each condition, on the left: jittered data points (in shades of blue) represent individual measurements and are color-coded according to biological replicate. Means of individual experiments are presented in black. Overall means ± SE are indicated in red. Histograms of all data points indicating the mean (solid line) as well as the 25th and 75th percentiles (dashed line) are shown to the right of the individual data. n = 4 independent experiments, analyzing 141,172,204,346 wildtype static-; 109,136,195,371 wildtype shear-; 83,142,147,377 KO clone 4 shear-; and 171,352,130,129 KO clone 6 shear-exposed cells. c, Representative micrographs for indicated conditions, scale-bar: 5 μm. Representative of 3 independent experiments. d, Ratio of phospho-tyrosine fluorescence at junctions (VE-cadherin-positive structures) and cytosol for individual cells. n = 3 independent experiments, analyzing 140,175,72 wildtype static-; 168,109,99 wildtype shear-; 73,89,72 KO clone 4 static-; 83,142,81 KO clone 4 shear-; 74,91,92 KO clone 6 static- and 103,143,38 KO clone 6 shear-exposed cells. P values are from one-way ANOVA of experimental means (b,d).
Extended Data Fig. 4 Defective mechanoresponses of β-II spectrin-KO cells persist in cells with high density of surface HA.
a, Untransfected endothelial cells and cells overexpressing HA-synthase 3 (HAS3-gfp) were incubated with fluorescent HA-binding complex (HABC) and imaged. Representative of 2 independent experiments. Here and elsewhere, scale-bar: 5 μm. b,c, Untransfected endothelial cells and cells overexpressing HA-synthase 3 (HAS3-gfp) were grown in collagen-coated microfluidic chambers under static conditions or subjected to a constant shear stress of 15 dynes/cm2 for 30 min, as indicated prior to fixation and phalloidin staining. b, Representative images of HAS-gfp (left panel and green in merge) and F-actin (middle panel and magenta in merge) for indicated conditions. c, Interquartile ranges (the ranges between the 25th to 75th percentile of the data) of the distribution of segmented filament orientations for the indicated conditions. Here and elsewhere, data are means ± SE, n = 3 independent experiments, each quantifying 12 images per condition. P values are from one-way ANOVA of experimental means (c).
Extended Data Fig. 5 Effect of GsMTx4 on hypotonically-induced [Ca2+] changes.
Live imaging of confluent endothelial cells expressing the cytosolic [Ca2+] indicator GCaMP6 before and after the introduction of hypotonic stress (grey background). Images were acquired every 12 s. Cells were treated with vehicle (PBS) or the small peptide GsMTx4 for 30 min prior to imaging. Quantification of mean GCaMP6 fluorescence over time. Data are means ± SE (shaded area), n = 3 independent experiments, quantifying 12,8,9 cells per condition.
Extended Data Fig. 6 Comparable expression of Piezo1 and caveolin-1 proteins in wildtype and β-II spectrin-KO cells.
a,b, Wild-type or spectrin-KO endothelial cells were lysed and a Piezo1 or b caveolin-1 expression was assessed by immunoblotting and compared to vinculin as in Fig. 6. Quantification of protein expression by densitometric analysis of n = 3 independent experiments, normalized in each instace to the wild-type control, presented as means ± SD. c, Ratio of apical/basal membrane fluorescence of caveolin-1. Data are means ± SE (shaded area) of 3 independent experiments, each analyzing 32,38,37 wildtype; 33,51,37 KO clone 4; 42,45,31 KO clone 6 cells. d, Confocal images of wild-type or spectrin-KO endothelial cells expressing cavin-2-RFP and caveolin-1-GFP. Representative of 3 independent experiments. Scale bars: 5 μm. e, Pearson’s coefficient assessing the correlation between cavin-2 and caveolin-1, calculated for multiple individual cells under indicated conditions. Data are means ± SE (shaded area), n = 4 independent experiments, each analyzing 8,11,8,9 wildtype isotonic- and 8,11,8,9 hypotonic-treated cells. P values are from one-way ANOVA (a–c) or Student’s t-test (e) of experimental means.
Extended Data Fig. 7 Effect of shear on surface-expression of platelet receptors.
Representative micrographs of indicated cells following exposure to 30 mins of shear stress. Immunostaining was performed on fixed unpermeabilized cells. Distribution of vWF (magenta) and P-selectin (green) are shown. Representative of 3 independent experiments. Scale bars: 5 μm.
Supplementary information
Source data
Source Data Fig. 1
Numerical source data for Fig. 1.
Source Data Fig. 2
Numerical source data for Fig. 2.
Source Data Fig. 2
Uncropped blots for Figs. 2.
Source Data Fig. 3
Numerical source data for Fig. 3
Source Data Fig. 3
Uncropped blots for Figs. 3.
Source Data Fig. 4
Numerical source data for Fig. 4.
Source Data Fig. 5
Numerical source data for Fig. 5.
Source Data Fig. 6
Numerical source data for Fig. 6.
Source Data Fig. 6
Uncropped blots for Fig. 6.
Source Data Fig. 7
Numerical source data for Fig. 7.
Source Data Fig. 7
Uncropped blots for Fig. 7.
Source Data Extended Data Fig. 1
Uncropped blots for Extended Data Fig. 1.
Source Data Extended Data Fig. 2
Numerical source data for Extended Data Fig. 2.
Source Data Extended Data Fig. 3
Numerical source data for Extended Data Fig. 3.
Source Data Extended Data Fig. 4
Numerical source data for Extended Data Fig. 4.
Source Data Extended Data Fig. 5
Numerical source data for Extended Data Fig. 5.
Source Data Extended Data Fig. 6
Numerical source data for Extended Data Fig. 6.
Rights and permissions
About this article
Cite this article
Mylvaganam, S., Plumb, J., Yusuf, B. et al. The spectrin cytoskeleton integrates endothelial mechanoresponses. Nat Cell Biol 24, 1226–1238 (2022). https://doi.org/10.1038/s41556-022-00953-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-022-00953-5
This article is cited by
-
Characterization of two distinct immortalized endothelial cell lines, EA.hy926 and HMEC-1, for in vitro studies: exploring the impact of calcium electroporation, Ca2+ signaling and transcriptomic profiles
Cell Communication and Signaling (2024)
-
Revisiting Virchow’s triad: exploring the cellular and molecular alterations in cerebral venous congestion
Cell & Bioscience (2024)
-
Tutorial: fluorescence lifetime microscopy of membrane mechanosensitive Flipper probes
Nature Protocols (2024)
-
Mechanisms of mechanotransduction and physiological roles of PIEZO channels
Nature Reviews Molecular Cell Biology (2024)
-
Mechanically induced topological transition of spectrin regulates its distribution in the mammalian cell cortex
Nature Communications (2024)