Abstract
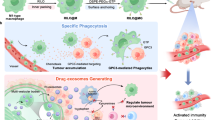
Most patients with cholangiocarcinoma (CCA) develop extrahepatic malignant biliary obstructions, which require palliative drainage to normalize bilirubin levels and to improve the patients’ overall survival. Here, we report that the infusion of methotrexate-containing plasma-membrane microvesicles derived from apoptotic human tumour cells into the bile-duct lumen of patients with extrahepatic CCA mobilized and activated neutrophils and relieved biliary obstruction in 25% of the patients. Neutrophil recruitment by the microvesicles was associated with an increase in uridine diphosphate glucose and complement C5, and led to the degradation of the stromal barrier of CCA. The microvesicles induced pyroptosis of CCA cells through a gasdermin E-dependent pathway, and their intracellular contents released upon CCA-cell death activated patient-derived macrophages into producing proinflammatory cytokines, which attracted a secondary wave of neutrophils to the tumour site. Our findings suggest a possible treatment for the alleviation of obstructive extrahepatic CCA with few adverse effects, and highlight the potential of tumour-cell-derived microvesicles as drug carriers for antitumour therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The raw and analysed datasets generated during the study are too large to be publicly shared, but are available for research purposes from the corresponding authors on reasonable request.
References
Razumilava, N. & Gores, G. J. Cholangiocarcinoma. Lancet 383, 2168–2179 (2014).
Doherty, B., Nambudiri, V. E. & Palmer, W. C. Update on the diagnosis and treatment of cholangiocarcinoma. Curr. Gastroenterol. Rep. 19, 2 (2017).
Wakai, T. et al. Surgical management of carcinoma in situ at ductal resection margins in patients with extrahepatic cholangiocarcinoma. Ann. Gastroenterol. Surg. 2, 359–366 (2018).
Ortner, M.-A. & Dorta, G. Technology insight: photodynamic therapy for cholangiocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 3, 459–467 (2006).
Blechacz, B., Komuta, M., Roskams, T. & Gores, G. J. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev. Gastroenterol. Hepatol. 8, 512–522 (2011).
Patel, T. Cholangiocarcinoma. Nat. Clin. Pract. Gastroenterol. Hepatol. 3, 33–42 (2006).
Banales, J. M. et al. Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 13, 261–280 (2016).
Artifon, E. L. A., Ferreira, F. C. & Sakai, P. Endoscopic ultrasound-guided biliary drainage. Korean J. Radiol. 13, S74–S82 (2012).
Ma, J. et al. Reversing drug resistance of soft tumor-repopulating cells by tumor cell-derived chemotherapeutic microparticles. Cell Res. 26, 713–727 (2016).
Ma, J. et al. Mechanisms by which dendritic cells present tumor microparticle antigens to CD8+ T cells. Cancer Immunol. Res. 6, 1057–1068 (2018).
Dong, W. et al. Oral delivery of tumor microparticle vaccines activates NOD2 signaling pathway in ileac epithelium rendering potent antitumor T cell immunity. Oncoimmunology. 6, e1282589 (2017).
Jin, X. et al. Pre-instillation of tumor microparticles enhances intravesical chemotherapy of nonmuscle-invasive bladder cancer through a lysosomal pathway. Biomaterials 113, 93–104 (2017).
Zhang, H. et al. Cell-free tumor microparticle vaccines stimulate dendritic cells via cGAS/STING signaling. Cancer Immunol. Res. 3, 196–205 (2015).
Mause, S. F. & Weber, C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ. Res. 107, 1047–1057 (2010).
Tang, K. et al. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat. Commun. 3, 1282 (2012).
Harada, K. & Nakanuma, Y. Biliary innate immunity: function and modulation. Mediators Inflamm. 2010, 373878 (2010).
Zhang, Q. et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104–107 (2010).
Albrengues, J. et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 361, eaao4227 (2018).
Sesma, J. I. et al. UDP-glucose promotes neutrophil recruitment in the lung. Purinergic Signal. 12, 627–635 (2016).
Arase, T. et al. The UDP-glucose receptor P2RY14 triggers innate mucosal immunity in the female reproductive tract by inducing IL-8. J. Immunol. 182, 7074–7084 (2009).
Sesma, J. I. et al. The UDP-sugar-sensing P2Y14 receptor promotes Rho-mediated signaling and chemotaxis in human neutrophils. Am. J. Physiol. Cell Physiol. 303, C490–C498 (2012).
Fairbanks, L. D. et al. Methotrexate inhibits the first committed step of purine biosynthesis in mitogen-stimulated human T-lymphocytes: a metabolic basis for efficacy in rheumatoid arthritis? Biochem. J. 342, 143–152 (1999).
Sadik, C. D., Miyabe, Y., Sezin, T. & Luster, A. D. The critical role of C5a as an initiator of neutrophil-mediated autoimmune inflammation of the joint and skin. Semin. Immunol. 37, 21–29 (2018).
Kew, R. R. & Webster, R. O. Gc-globulin (vitamin D-binding protein) enhances the neutrophil chemotactic activity of C5a and C5a des Arg. J Clin Invest. 82, 364–369 (1988).
Wang, Y. et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547, 99–103 (2017).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
Ding, J. et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116 (2016).
Ruhl, S. et al. ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science 362, 956–960 (2018).
Yuan, J., Amin, P. & Ofengheim, D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat. Rev. Neurosci. 20, 19–33 (2019).
Lin, J. et al. RIPK1 counteracts ZBP1-mediated necroptosis to inhibit inflammation. Nature 540, 124–128 (2016).
Vande Walle, L. & Lamkanfi, M. Pyroptosis. Curr. Biol. 26, R568–R572 (2016).
Sun, Y. et al. Chemotherapeutic tumor microparticles combining low-dose irradiation reprogram tumor-promoting macrophages through a tumor-repopulating cell-curtailing pathway. Oncoimmunology 6, e1309487 (2017).
Ma, R. et al. Tumor cell-derived microparticles polarize M2 tumor-associated macrophages for tumor progression. Oncoimmunology. 5, e1118599 (2016).
Pylaeva, E., Lang, S. & Jablonska, J. The essential role of type I interferons in differentiation and activation of tumor-associated neutrophils. Front. Immunol. 7, 629 (2016).
Shaul, M. E. & Fridlender, Z. G. Neutrophils as active regulators of the immune system in the tumor microenvironment. J. Leukoc. Biol. 102, 343–349 (2017).
El-Benna, J. et al. Priming of the neutrophil respiratory burst: role in host defense and inflammation. Immunol. Rev. 273, 180–193 (2016).
Stojkov, D. et al. ROS and glutathionylation balance cytoskeletal dynamics in neutrophil extracellular trap formation. J. Cell Biol. 216, 4073–4090 (2017).
Delgado-Rizo, V. et al. Neutrophil extracellular traps and its implications in inflammation: an overview. Front. Immunol. 8, 81 (2017).
Fridlender, Z. G. & Albelda, S. M. Tumor-associated neutrophils: friend or foe? Carcinogenesis. 33, 949–955 (2012).
Kolaczkowska, E. & Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175 (2013).
Vols, S., Sionov, R. V. & Granot, Z. Always look on the bright side: anti-tumor functions of neutrophils. Curr. Pharm. Des. 23, 4862–4892 (2017).
Bergsbaken, T., Fink, S. L. & Cookson, B. T. Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 7, 99–109 (2009).
Long, K. B. et al. IFNγ and CCL2 cooperate to redirect tumor-infiltrating monocytes to degrade fibrosis and enhance chemotherapy efficacy in pancreatic carcinoma. Cancer Discov. 6, 400–413 (2016).
Mertens, J. C. & Gores, G. J. Targeting tumor stroma: exploiting apoptotic priming. Oncotarget 3, 1501–1502 (2012).
Acknowledgements
This work was supported by National Natural Science Foundation of China (81788101, 81661128007, 81530080, 81773062 and 91942314), the Chinese Academy of Medical Sciences Initiative for Innovative Medicine (CAMS-I2M) (2017-I2M-1-001 and 2016-I2M-1-007).
Author information
Authors and Affiliations
Contributions
B.H., Y.L. and X.W. conceived the project. Y.L., Yunfeng Gao, Hui Zhang, N.Z., P.X., J.W., Yuan Gao, X.J., X.L., J.L., Y.Z., K.T., J.M., Huafeng Zhang and J.X. performed the experiments. B.H., Y.L., F.Y., W.T. and X.W. developed methodology. B.H., Y.L., Yunfeng Gao, Hui Zhang and X.W. performed data analysis. B.H. and Y.L. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
B.H. is the inventor on patent no. ZL201110241369.8, owned by Hubei Soundny (Sheng-Qi-An) Biotech, which covers the preparation and use of drug-packaging tumour-cell-derived microparticles in cancer therapies. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures and tables.
Supplementary Methods
Clinical research protocol.
Supplementary Methods
Appendix to clinical research protocol.
Rights and permissions
About this article
Cite this article
Gao, Y., Zhang, H., Zhou, N. et al. Methotrexate-loaded tumour-cell-derived microvesicles can relieve biliary obstruction in patients with extrahepatic cholangiocarcinoma. Nat Biomed Eng 4, 743–753 (2020). https://doi.org/10.1038/s41551-020-0583-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-020-0583-0