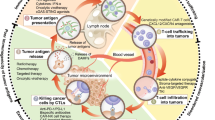
Abstract
Checkpoint-inhibitor (CPI) immunotherapy has achieved remarkable clinical success, yet its efficacy in ‘immunologically cold’ tumours has been modest. Interleukin-12 (IL-12) is a powerful cytokine that activates the innate and adaptive arms of the immune system; however, the administration of IL-12 has been associated with immune-related adverse events. Here we show that, after intravenous administration of a collagen-binding domain fused to IL-12 (CBD–IL-12) in mice bearing aggressive mouse tumours, CBD–IL-12 accumulates in the tumour stroma due to exposed collagen in the disordered tumour vasculature. In comparison with the administration of unmodified IL-12, CBD–IL-12 induced sustained intratumoural levels of interferon-γ, substantially reduced its systemic levels as well as organ damage and provided superior anticancer efficacy, eliciting complete regression of CPI-unresponsive breast tumours. Furthermore, CBD–IL-12 potently synergized with CPI to eradicate large established melanomas, induced antigen-specific immunological memory and controlled tumour growth in a genetically engineered mouse model of melanoma. CBD–IL-12 may potentiate CPI immunotherapy for immunologically cold tumours.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the results in this study are provided within the Article and its Supplementary Information. All data generated in this study, including source data and the data used to make the figures, are available from Figshare at https://doi.org/10.6084/m9.figshare.11971371.
References
Larkin, J. et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373, 23–34 (2015).
Sharma, P. & Allison, J. P. The future of immune checkpoint therapy. Science 348, 56–61 (2015).
Rosenberg, S. A., Yang, J. C. & Restifo, N. P. Cancer immunotherapy: moving beyond current vaccines. Nat. Med. 10, 909–915 (2004).
Langrish, C. L. et al. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol. Rev. 202, 96–105 (2004).
McHeyzer-Williams, M. G. et al. Antigen-specific immunity. Immunol. Res. 22, 223–236 (2000).
Fallarino, F., Ashikari, A., Boon, T. & Gajewski, T. F. Antigen-specific regression of established tumors induced by active immunization with irradiated IL-12- but not B7-1-transfected tumor cells. Int. Immunol. 9, 1259–1269 (1997).
Rogge, L. et al. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J. Exp. Med. 185, 825–831 (1997).
Tugues, S. et al. New insights into IL-12-mediated tumor suppression. Cell Death Differ. 22, 237–246 (2015).
Lasek, W., Zagozdzon, R. & Jakobisiak, M. Interleukin 12: still a promising candidate for tumor immunotherapy? Cancer Immunol. Immunother. 63, 419–435 (2014).
Kerkar, S. P. et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J. Clin. Investig. 121, 4746–4757 (2011).
Momin, N. et al. Anchoring of intratumorally administered cytokines to collagen safely potentiates systemic cancer immunotherapy. Sci. Transl. Med. 11, eaaw2614 (2019).
Ishihara, J. et al. Targeted antibody and cytokine cancer immunotherapies through collagen affinity. Sci. Transl. Med. 11, eaau3259 (2019).
Bekaii-Saab, T. S. et al. A phase I trial of paclitaxel and trastuzumab in combination with interleukin-12 in patients with HER2/neu-expressing malignancies. Mol. Cancer Ther. 8, 2983–2991 (2009).
Atkins, M. B. et al. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin. Cancer Res. 3, 409–417 (1997).
Gollob, J. A. et al. Phase I trial of concurrent twice-weekly recombinant human interleukin-12 plus low-dose IL-2 in patients with melanoma or renal cell carcinoma. J. Clin. Oncol. 21, 2564–2573 (2003).
Mariathasan, S. et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548 (2018).
Zhang, Y., Li, N., Suh, H. & Irvine, D. J. Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat. Commun. 9, 6 (2018).
Joyce, J. A. & Fearon, D. T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 348, 74–80 (2015).
Gollob, J. A. et al. Phase I trial of twice-weekly intravenous interleukin 12 in patients with metastatic renal cell cancer or malignant melanoma: ability to maintain IFN-γ induction is associated with clinical response. Clin. Cancer Res. 6, 1678–1692 (2000).
Ayers, M. et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Invest. 127, 2930–2940 (2017).
Spranger, S., Dai, D., Horton, B. & Gajewski, T. F. Tumor-residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell 31, 711–723 (2017).
Wang, L. L. et al. CXC-chemokine-ligand-10 gene therapy efficiently inhibits the growth of cervical carcinoma on the basis of its anti-angiogenic and antiviral activity. Biotechnol. Appl. Biochem. 53, 209–216 (2009).
Ghiringhelli, F. et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β-dependent adaptive immunity against tumors. Nat. Med. 15, 1170–1178 (2009).
Liu, F. & Whitton, J. L. Cutting edge: re-evaluating the in vivo cytokine responses of CD8+ T cells during primary and secondary viral infections. J. Immunol. 174, 5936–5940 (2005).
Ryffel, B. Interleukin-12: role of interferon-γ in IL-12 adverse effects. Clin. Immunol. Immunopathol. 83, 18–20 (1997).
Neri, D. Antibody-cytokine fusions: versatile products for the modulation of anticancer immunity. Cancer Immunol. Res. 7, 348–354 (2019).
Zhou, F. Molecular mechanisms of IFN-γ to up-regulate MHC class I antigen processing and presentation. Int. Rev. Immunol. 28, 239–260 (2009).
Clatza, A., Bonifaz, L. C., Vignali, D. A. & Moreno, J. CD40-induced aggregation of MHC class II and CD80 on the cell surface leads to an early enhancement in antigen presentation. J. Immunol. 171, 6478–6487 (2003).
Ho, P. C. et al. Immune-based antitumor effects of BRAF inhibitors rely on signaling by CD40L and IFNγ. Cancer Res. 74, 3205–3217 (2014).
Pan, D. et al. A major chromatin regulator determines resistance of tumor cells to T cell-mediated killing. Science 359, 770–775 (2018).
Boross, P. et al. Anti-tumor activity of human IgG1 anti-gp75 TA99 mAb against B16F10 melanoma in human FcgammaRI transgenic mice. Immunol. Lett. 160, 151–157 (2014).
Castle, J. C. et al. Exploiting the mutanome for tumor vaccination. Cancer Res. 72, 1081–1091 (2012).
Dankort, D. et al. Braf V600E cooperates with Pten loss to induce metastatic melanoma. Nat. Genet. 41, 544–552 (2009).
Ishihara, J. et al. Matrix-binding checkpoint immunotherapies enhance antitumor efficacy and reduce adverse events. Sci. Transl. Med. 9, eaan0401 (2017).
Gros, A. et al. PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. J. Clin. Investig. 124, 2246–2259 (2014).
Spranger, S., Bao, R. & Gajewski, T. F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 523, 231–235 (2015).
Drake, C. G., Lipson, E. J. & Brahmer, J. R. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nat. Rev. Clin. Oncol. 11, 24–37 (2014).
Moynihan, K. D. et al. Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat. Med. 22, 1402–1410 (2016).
Garris, C. S. et al. Successful anti-PD-1 cancer immunotherapy requires T cell-dendritic cell crosstalk involving the cytokines IFN-γ and IL-12. Immunity 49, 1148–1161 (2018).
Coughlin, C. M. et al. Tumor cell responses to IFNγ affect tumorigenicity and response to IL-12 therapy and antiangiogenesis. Immunity 9, 25–34 (1998).
Nagy, J. A., Chang, S. H., Dvorak, A. M. & Dvorak, H. F. Why are tumour blood vessels abnormal and why is it important to know? Br. J. Cancer 100, 865–869 (2009).
Leonard, J. P. et al. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-γ production. Blood 90, 2541–2548 (1997).
Grigorian, A. & O’Brien, C. B. Hepatotoxicity secondary to chemotherapy. J. Clin. Transl. Hepatol. 2, 95–102 (2014).
van Herpen, C. M. et al. Intratumoral recombinant human interleukin-12 administration in head and neck squamous cell carcinoma patients modifies locoregional lymph node architecture and induces natural killer cell infiltration in the primary tumor. Clin. Cancer Res. 11, 1899–1909 (2005).
Mahvi, D. M. et al. Intratumoral injection of IL-12 plasmid DNA–results of a phase I/IB clinical trial. Cancer Gene Ther. 14, 717–723 (2007).
Swartz, M. A. & Lund, A. W. Lymphatic and interstitial flow in the tumour microenvironment: linking mechanobiology with immunity. Nat. Rev. Cancer 12, 210–219 (2012).
Halin, C. et al. Enhancement of the antitumor activity of interleukin-12 by targeted delivery to neovasculature. Nat. Biotechnol. 20, 264–269 (2002).
Fallon, J. et al. The immunocytokine NHS-IL12 as a potential cancer therapeutic. Oncotarget 5, 1869–1884 (2014).
Strauss, J. et al. First-in-human phase I trial of a tumor-targeted cytokine (NHS-IL12) in subjects with metastatic solid tumors. Clin. Cancer Res. 25, 99–109 (2019).
Hank, J. A. et al. Immunogenicity of the Hu14.18-IL2 immunocytokine molecule in adults with melanoma and children with neuroblastoma. Clin. Cancer Res. 15, 5923–5930 (2009).
Williford, J. M. et al. Recruitment of CD103+ dendritic cells via tumor-targeted chemokine delivery enhances efficacy of checkpoint inhibitor immunotherapy. Sci. Adv. 5, eaay1357 (2019).
Sasaki, K. et al. Engineered collagen-binding serum albumin as a drug conjugate carrier for cancer therapy. Sci. Adv. 5, eaaw6081 (2019).
Ishihara, J. et al. Improving efficacy and safety of agonistic anti-CD40 antibody through extracellular matrix affinity. Mol. Cancer Ther. 17, 2399–2411 (2018).
Acknowledgements
We thank T. Gajewski (University of Chicago) for sharing Tyr:creER+ LSL-BrafV600E Ptenfl/fl and Tyr:creER+ LSL-BrafV600E Ptenfl/fl Ctnnb1STA mice; A. Solanki for assistance with tail vein injections; S. Gomes for assistance with experiments; T. Li for assistance with histology analysis; staff at the Human Tissue Resource Center of the University of Chicago for assistance with organ sectioning; and D. Leclerc (University of Chicago Flow Cytometry Core) for assistance with Luminex assays.
Author information
Authors and Affiliations
Contributions
A.M., J.I., M.A.S. and J.A.H. designed the experiments and wrote the manuscript. A.M. and J.I. performed the experiments. P.H. assisted with the pulmonary metastasis model. L.P. assisted with the autochthonous melanoma model. T.M.M. assisted with the antigen restimulation experiment and prepared B16F10 exosomes. A.I. blindly evaluated histological sections. J.M.-W. and L.T.G. assisted with tumour experiments. A.T.A. assisted with blood chemistry analysis. M.M.R. assisted with and analysed MALDI-TOF data.
Corresponding authors
Ethics declarations
Competing interests
J.I., A.I., M.A.S. and J.A.H. are inventors on U.S. Provisional Patent applications 62/638,520, 28/984,351 and 62/727,156. J.I., A.I., M.A.S. and J.A.H. are founders and shareholders in Arrow Immune Inc., which is developing the technology presented in this report, and M.A.S. and J.A.H. have leadership roles in that company. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–13.
Rights and permissions
About this article
Cite this article
Mansurov, A., Ishihara, J., Hosseinchi, P. et al. Collagen-binding IL-12 enhances tumour inflammation and drives the complete remission of established immunologically cold mouse tumours. Nat Biomed Eng 4, 531–543 (2020). https://doi.org/10.1038/s41551-020-0549-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-020-0549-2
This article is cited by
-
Lipid-based nanosystems: the next generation of cancer immune therapy
Journal of Hematology & Oncology (2024)
-
Understanding the matrix: collagen modifications in tumors and their implications for immunotherapy
Journal of Translational Medicine (2024)
-
RNA nanotherapeutics with fibrosis overexpression and retention for MASH treatment
Nature Communications (2024)
-
Local delivery of cell surface-targeted immunocytokines programs systemic antitumor immunity
Nature Immunology (2024)
-
Anti-PD-1 cis-delivery of low-affinity IL-12 activates intratumoral CD8+T cells for systemic antitumor responses
Nature Communications (2024)