Abstract
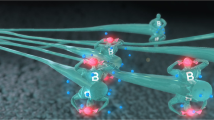
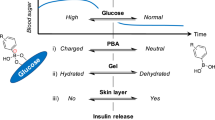
Glucose-responsive insulin delivery systems that mimic pancreatic endocrine function could enhance health and improve quality of life for people with type 1 and type 2 diabetes with reduced β-cell function. However, insulin delivery systems with rapid in vivo glucose-responsive behaviour typically have limited insulin-loading capacities and cannot be manufactured easily. Here, we show that a single removable transdermal patch, bearing microneedles loaded with insulin and a non-degradable glucose-responsive polymeric matrix, and fabricated via in situ photopolymerization, regulated blood glucose in insulin-deficient diabetic mice and minipigs (for minipigs >25 kg, glucose regulation lasted >20 h with patches of ~5 cm2). Under hyperglycaemic conditions, phenylboronic acid units within the polymeric matrix reversibly form glucose–boronate complexes that—owing to their increased negative charge—induce the swelling of the polymeric matrix and weaken the electrostatic interactions between the negatively charged insulin and polymers, promoting the rapid release of insulin. This proof-of-concept demonstration may aid the development of other translational stimuli-responsive microneedle patches for drug delivery.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The authors declare that all of the data supporting the findings of this study are available within the paper and the Supplementary Information.
References
Veiseh, O., Tang, B. C., Whitehead, K. A., Anderson, D. G. & Langer, R. Managing diabetes with nanomedicine: challenges and opportunities. Nat. Rev. Drug Discov. 14, 45–57 (2015).
Ohkubo, Y. et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res. Clin. Pract. 28, 103–117 (1995).
Owens, D. R., Zinman, B. & Bolli, G. B. Insulins today and beyond. Lancet 358, 739–746 (2001).
Bakh, N. A. et al. Glucose-responsive insulin by molecular and physical design. Nat. Chem. 9, 937–943 (2017).
Yu, J., Zhang, Y., Bomba, H. & Gu, Z. Stimuli‐responsive delivery of therapeutics for diabetes treatment. Bioeng. Transl. Med. 1, 323–337 (2016).
Ravaine, V., Ancla, C. & Catargi, B. Chemically controlled closed-loop insulin delivery. J. Control. Release 132, 2–11 (2008).
Wu, Q., Wang, L., Yu, H., Wang, J. & Chen, Z. Organization of glucose-responsive systems and their properties. Chem. Rev. 111, 7855–7875 (2011).
Yu, J. et al. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc. Natl Acad. Sci. USA 112, 8260–8265 (2015).
Yu, J. et al. Hypoxia and H2O2 dual-sensitive vesicles for enhanced glucose-responsive insulin delivery. Nano Lett. 17, 733–739 (2017).
Kost, J., Leong, K. & Langer, R. Ultrasound-enhanced polymer degradation and release of incorporated substances. Proc. Natl Acad. Sci. USA 86, 7663–7666 (1989).
Podual, K., Doyle, F. J. III & Peppas, N. A. Glucose-sensitivity of glucose oxidase-containing cationic copolymer hydrogels having poly (ethylene glycol) grafts. J. Control. Release 67, 9–17 (2000).
Podual, K., Doyle Iii, F. & Peppas, N. Preparation and dynamic response of cationic copolymer hydrogels containing glucose oxidase. Polymer 41, 3975–3983 (2000).
Gu, Z. et al. Injectable nano-network for glucose-mediated insulin delivery. ACS Nano 7, 4194–4201 (2013).
Brownlee, M. & Cerami, A. A glucose-controlled insulin-delivery system: semisynthetic insulin bound to lectin. Science 206, 1190–1191 (1979).
Brownlee, M. & Cerami, A. Glycosylated insulin complexed to concanavalin A: biochemical basis for a closed-loop insulin delivery system. Diabetes 32, 499–504 (1983).
Wang, C. et al. Red blood cells for glucose‐responsive insulin delivery. Adv. Mater. 29, 1606617 (2017).
Yang, R. et al. A glucose-responsive insulin therapy protects animals against hypoglycemia. JCI Insight 3, 97476 (2018).
Wang, J. et al. Charge-switchable polymeric complex for glucose-responsive insulin delivery in mice and pigs. Sci. Adv. 5, eaaw4357 (2019).
Chou, D. H.-C. et al. Glucose-responsive insulin activity by covalent modification with aliphatic phenylboronic acid conjugates. Proc. Natl Acad. Sci. USA 112, 2401–2406 (2015).
Matsumoto, A. et al. Synthetic “smart gel” provides glucose-responsive insulin delivery in diabetic mice. Sci. Adv. 3, eaaq0723 (2017).
Matsumoto, A., Yoshida, R. & Kataoka, K. Glucose-responsive polymer gel bearing phenylborate derivative as a glucose-sensing moiety operating at the physiological pH. Biomacromolecules 5, 1038–1045 (2004).
Kataoka, K., Miyazaki, H., Bunya, M., Okano, T. & Sakurai, Y. Totally synthetic polymer gels responding to external glucose concentration: their preparation and application to on–off regulation of insulin release. J. Am. Chem. Soc. 120, 12694–12695 (1998).
Matsumoto, A. et al. A synthetic approach toward a self‐regulated insulin delivery system. Angew. Chem. Int. Ed. 51, 2124–2128 (2012).
Mo, R., Jiang, T., Di, J., Tai, W. & Gu, Z. Emerging micro- and nanotechnology based synthetic approaches for insulin delivery. Chem. Soc. Rev. 43, 3595–3629 (2014).
Ye, T. et al. Tailoring the glucose-responsive volume phase transition behaviour of Ag@poly(phenylboronic acid) hybrid microgels: from monotonous swelling to monotonous shrinking upon adding glucose at physiological pH. Polym. Chem. 5, 2352–2362 (2014).
Wu, W., Mitra, N., Yan, E. C. & Zhou, S. Multifunctional hybrid nanogel for integration of optical glucose sensing and self-regulated insulin release at physiological pH. ACS Nano 4, 4831–4839 (2010).
Wu, X. et al. Selective sensing of saccharides using simple boronic acids and their aggregates. Chem. Soc. Rev. 42, 8032–8048 (2013).
Brooks, W. L. & Sumerlin, B. S. Synthesis and applications of boronic acid-containing polymers: from materials to medicine. Chem. Rev. 116, 1375–1397 (2015).
Sullivan, S. P. et al. Dissolving polymer microneedle patches for influenza vaccination. Nat. Med. 16, 915–920 (2010).
Sullivan, S. P., Murthy, N. & Prausnitz, M. R. Minimally invasive protein delivery with rapidly dissolving polymer microneedles. Adv. Mater. 20, 933–938 (2008).
Prausnitz, M. R. Microneedles for transdermal drug delivery. Adv. Drug Deliv. Rev. 56, 581–587 (2004).
Vancoillie, G. & Hoogenboom, R. Synthesis and polymerization of boronic acid containing monomers. Polym. Chem. 7, 5484–5495 (2016).
Ding, Z., Guan, Y., Zhang, Y. & Zhu, X. Layer-by-layer multilayer films linked with reversible boronate ester bonds with glucose-sensitivity under physiological conditions. Soft Matter 5, 2302–2309 (2009).
Hisamitsu, I., Kataoka, K., Okano, T. & Sakurai, Y. Glucose-responsive gel from phenylborate polymer and poly(vinyl alcohol): prompt response at physiological pH through the interaction of borate with amino group in the gel. Pharm. Res. 14, 289–293 (1997).
Summerfield, A., Meurens, F. & Ricklin, M. E. The immunology of the porcine skin and its value as a model for human skin. Mol. Immunol. 66, 14–21 (2015).
Larsen, M. O. et al. Mild streptozotocin diabetes in the Göttingen minipig. A novel model of moderate insulin deficiency and diabetes. Am. J. Physiol. Endocrinol. Metab. 282, E1342–E1351 (2002).
Larsen, M. O., Rolin, B., Wilken, M., Carr, R. D. & Gotfredsen, C. F. Measurements of insulin secretory capacity and glucose tolerance to predict pancreatic β-cell mass in vivo in the nicotinamide/streptozotocin Göttingen minipig, a model of moderate insulin deficiency and diabetes. Diabetes 52, 118–123 (2003).
Akhtar, M. S., Ramzan, A., Ali, A. & Ahmad, M. Effect of Amla fruit (Emblica officinalis Gaertn.) on blood glucose and lipid profile of normal subjects and type 2 diabetic patients. Int. J. Food Sci. Nutr. 62, 609–616 (2011).
Larsen, M. O. & Rolin, B. Use of the Göttingen minipig as a model of diabetes, with special focus on type 1 diabetes research. ILAR J. 45, 303–313 (2004).
Larsen, M. et al. The conscious Göttingen minipig as a model for studying rapid pulsatile insulin secretion in vivo. Diabetologia 45, 1389–1396 (2002).
Prausnitz, M. R. & Langer, R. Transdermal drug delivery. Nat. Biotechnol. 26, 1261–1268 (2008).
Zhang, Y. et al. Advances in transdermal insulin delivery. Adv. Drug Deliv. Rev. 139, 51–70 (2018).
Yu, J., Zhang, Y., Kahkoska, A. R. & Gu, Z. Bioresponsive transcutaneous patches. Curr. Opin. Biotechnol. 48, 28–32 (2017).
Lu, Y., Aimetti, A. A., Langer, R. & Gu, Z. Bioresponsive materials. Nat. Rev. Mater. 2, 16075 (2017).
Kaarsholm, N. C. et al. Engineering glucose responsiveness into insulin. Diabetes 67, 299–308 (2018).
Yang, S. et al. Phase‐transition microneedle patches for efficient and accurate transdermal delivery of insulin. Adv. Funct. Mater. 25, 4633–4641 (2015).
Gu, Z. & Wang, J. Charge-switchable polymeric depot for glucose-triggered insulin delivery with ultrafast response. Patent no. WO2019104006A1 (2017).
Wang, J. et al. Glucose transporter inhibitor-conjugated insulin mitigates hypoglycemia. Proc. Natl Acad. Sci. USA 116, 10744–10748 (2019).
Chen, W. et al. Microneedle-array patches loaded with dual mineralized protein/peptide particles for type 2 diabetes therapy. Nat. Commun. 8, 1777 (2017).
Yu, J. et al. Insulin‐responsive glucagon delivery for prevention of hypoglycemia. Small 13, 1603028 (2017).
Acknowledgements
This work was supported by grants from Zenomics Inc. and a start-up package from University of California, Los Angeles. We acknowledge the use of the Analytical Instrumentation Facility at North Carolina State, which is supported by the State of North Carolina and National Science Foundation. A.R.K. is supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (grant no. F30DK113728). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
J.Y. and Z.G. conceived the study idea. J.Y., A.R.K., J.B.B., R.L. and Z.G. designed the experiments. J.Y., J.W., Y.Z., G.C., W.M. and Y.Y. performed the experiments. All authors contributed to writing the manuscript, discussing the results and implications and editing the manuscript at all stages.
Corresponding author
Ethics declarations
Competing interests
Z.G., J.Y. and G.C. have applied for patents related to this study. Z.G. is a scientific co-founder of Zenomics Inc. R.L. and J.B.B. are Scientific Advisory Board members of Zenomics Inc. J.Y., Y.Z., W.M. and Y.Y. are full-time employees of Zenomics Inc. R.L. discloses potential competing interests due to his affiliation with Zenomics Inc. For a list of entities with which R.L. is involved, compensated or uncompensated, see https://tinyurl.com/RLCOINBME. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–15 and Tables 1–5.
Rights and permissions
About this article
Cite this article
Yu, J., Wang, J., Zhang, Y. et al. Glucose-responsive insulin patch for the regulation of blood glucose in mice and minipigs. Nat Biomed Eng 4, 499–506 (2020). https://doi.org/10.1038/s41551-019-0508-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-019-0508-y
This article is cited by
-
Microenvironment-responsive nanomedicines: a promising direction for tissue regeneration
Military Medical Research (2024)
-
An orally administered glucose-responsive polymeric complex for high-efficiency and safe delivery of insulin in mice and pigs
Nature Nanotechnology (2024)
-
Endogenous stimuli-responsive separating microneedles to inhibit hypertrophic scar through remodeling the pathological microenvironment
Nature Communications (2024)
-
Digital automation of transdermal drug delivery with high spatiotemporal resolution
Nature Communications (2024)
-
In vivo gene editing of T-cells in lymph nodes for enhanced cancer immunotherapy
Nature Communications (2024)