Abstract
Nipah virus infection, one of the top priority diseases recognized by the World Health Organization, underscores the urgent need to develop effective countermeasures against potential epidemics and pandemics. Here, we identify a fully human single-domain antibody that targets a highly conserved cryptic epitope situated at the dimeric interface of the Nipah virus G protein (receptor binding protein, RBP), as elucidated through structures by high-resolution cryo-electron microscopy (cryo-EM). This unique binding mode disrupts the tetramerization of the G protein, consequently obstructing the activation of the F protein and inhibiting viral membrane fusion. Furthermore, our investigations reveal that this compact antibody displays enhanced permeability across the blood-brain barrier (BBB) and demonstrates superior efficacy in eliminating pseudovirus within the brain in a murine model of Nipah virus infection, particularly compared to the well-characterized antibody m102.4 in an IgG1 format. Consequently, this single-domain antibody holds promise as a therapeutic candidate to prevent Nipah virus infections and has potential implications for vaccine development.
Similar content being viewed by others
Introduction
Nipah virus (NiV) is an emerging zoonotic pathogen that can cause severe respiratory illness and fatal encephalitis in humans1,2,3. Owing to its high mortality rate (50-95%)4 and potential for person-to-person transmission, NiV is among the priority pathogens of epidemic and pandemic potential according to the World Health Organization5, and there is an urgent need for the development of effective treatments and vaccines.
A key element in combating NiV infections is the development of NiV-neutralizing antibodies. We previously developed the monoclonal antibody (mAb) m102.4. This mAb demonstrated potent neutralization against NiV and its closely related counterpart, Hendra virus (HeV), both of which are classified within the genus Henipavirus6,7,8,9. Previous studies showed that m102.4 neutralized NiV better than HeV despite being originally selected against sGHeV10. This antibody has completed phase 1 clinical trials and has successfully been used to treat 16 individuals as an emergency postexposure therapy on a compassionate basis11. Recently, several additional NiV-neutralizing antibodies targeting different epitopes on the henipavirus G glycoprotein have also been reported12,13. However, an analysis of the locations of these epitopes revealed that certain amino acids within the epitopes are not conserved among HeV and different NiV isolates4,12,13,14 (Fig. 1a; Supplementary Fig. 1). Therefore, the identification of new NiV-neutralizing antibodies and the elucidation of their epitopes are crucial in advancing ongoing efforts to address the urgent global health challenge posed by NiV and related pathogens.
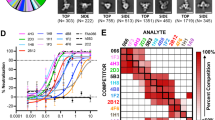
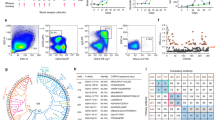
a Neutralizing mAbs (m102.4 (PDB ID: 6CMG), HENV-26 (PDB ID: 6VY6), HENV-32 (PDB ID: 6VY4), hAH1.3 (PDB ID: 7SYY), and nAH1.3 (PDB ID: 7TXZ)) representing four different antigenic binding sites are mapped onto the surface of the HeV-RBP or NiV-RBP head domain surfaces. The surfaces of the HeV-RBP or NiV-RBP head domain are colored gray, with nonconserved amino acids between NiV and HeV highlighted in red based on the sequence alignment in Supplementary Fig. 1. b Schematic illustration of the antibody panning process for the NiV or HeV G protein. The schemes were created with BioRender.com under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license. c Identification of G protein binders. Using a high-throughput screening ELISA, the binding activity of 1000 randomly selected sequences from the output libraries after 4 rounds of selection was calculated. d Binding kinetics of UdAb n425 to the immobilized NiV-G head domain determined by biolayer interferometry. The n425 concentrations used were 100 nM (red), 33 nM (green), 11 nM (blue), 3.7 nM (purple), and 1.2 nM (orange). The vertical dashed line corresponds to the transition between the association and dissociation phases. Curve fitting was performed to extrapolate equilibrium dissociation constant values using a 1:1 global model. e Binding and neutralizing activity of n425 against NiV or HeV pseudoviruses. The negative control (gray) is a neutralizing UdAb against the SARS-CoV-2 RBD. The data are presented as the mean ± standard deviation (S.D.); n = 2 biologically independent experiments for ELISA binding, n = 3 biologically independent experiments for henipavirus pseudovirus neutralization. Source data are provided as a Source Data File.
Recently, using a CDR grafting approach, we successfully grafted human naïve complementarity-determining regions (CDRs) into a soluble and stable scaffold based on the framework regions of a heavy-chain variable region allele. Due to their small size and fully human origin, this novel antibody format was designated as fully human single-domain antibodies (UdAbs). As a proof of concept, we demonstrated that these high-affinity UdAbs are capable of targeting cryptic epitopes in diverse antigens15,16,17,18.
In this work, we utilize this platform to discover a unique NiV-neutralizing UdAb that specifically targets a conserved cryptic epitope located at the dimeric interface of the NiV G glycoprotein. Compared to the full-length monoclonal antibody m102.4, this single-domain antibody displays significantly greater potency in inhibiting viral membrane fusion and more efficient penetration into the murine brain. These findings not only deepen our insights into viral neutralization mechanisms but also hold promise for the development of broad-spectrum therapeutics and vaccines against henipavirus.
Results
Identification of a broadly neutralizing human single-domain antibody against henipavirus
To identify novel antibodies with broad neutralizing potential, we first used the HeV G protein as the antigen for panning against the phage-displayed UdAb library (Fig. 1b). Subsequently, we conducted sequential screening against the NiV G protein through an ELISA-based high-throughput screening assay (Fig. 1c). A single-domain antibody, designated n425, exhibited superior biophysical properties and potent binding to henipavirus G proteins at low nanomolar concentrations, as determined by biolayer interferometry (Fig. 1d, e; Supplementary Fig. 2). Moreover, it demonstrated extensive neutralization against HeV and two distinct strains of NiV, namely, Bangladesh (NiVB) and Malaysia (NiVM).
UdAb n425 recognizes a novel epitope outside the receptor binding site
To explore the binding epitope of n425, we conducted a competitive binding assay with previously reported NiV-neutralizing antibodies4,6,12,13. Notably, n425 displayed only intermediate competition with HENV-32 and did not exhibit any competition with the other four antibodies tested (Fig. 2a; Supplementary Fig. 3). We also examined the synergy of neutralization by n425 and other antibodies and found that n425, in combination with m102.4, demonstrated the most potent synergistic effect (Fig. 2b, c). Since m102.4 neutralizes NiV by competitively inhibiting G protein-mediated viral attachment to the host receptor, these data unequivocally suggest that UdAb n425 is a noncompeting antibody for receptor binding, employing a mechanism distinct from that of m102.4 in viral neutralization. Since the competition binding assay indicated that HENV-32 shares competing epitope with n425, we further examined whether synergistic effects exist when combining HENV-32 with m102.4. Indeed, comparable enhancement in neutralizing activity was observed when m102.4 was combined with either HENV-32 or n425 (Supplementary Fig. 4).
a Competition of n425 with previously reported neutralizing mAbs or the receptor Ephrin B2 for NiV-G protein binding, as measured by biolayer interferometry. Values are the percentage of binding that occurred during competition compared to noncompeted binding, which was normalized to 100%. A residual binding percentage greater than 70% indicates noncompeting pairs, 30–69% indicates intermediate competition and less than 30% indicates strongly competing pairs. See also Supplymentary Fig. 3. b Neuralization of henipavirus pseudovirus by individual n425, individual mAbs, including m102.4, HENV-32, nAH1.3, and hAH1.3, and a cocktail of n425 and one mAb. Data represent the mean ± S.D. (n = 3 biologically independent cells). c Combination index (CI) values were calculated using CompuSyn software and determined by comparing the IC50 of the antibody alone to that of the combination of two antibodies. Source data are provided as a Source Data File.
Structures of UdAb n425 in complex with the NiV G protein
To unveil the neutralization mechanism of n425 and provide a blueprint for vaccine design, we performed cryo-electron microscopy (cryo-EM) structural study on UdAb n425 in complex with the ectodomain of NiV G proteins. Previous studies have reported that the NiV-G ectodomain adopts a tetrameric structure12. Cryo-EM analysis revealed that when n425 forms complexes with NiVM G or NiVB G(S76C), particles became heterogenous, including different oligomer states (Supplementary Fig. 5, 7). The structures of the NiVM G ectodomain monomer complexed with n425 and the NiVB G(S76C) monomer complexed with n425 were determined to the overall resolution of 3.63 Å and 3.22 Å, respectively (Supplementary Fig. 5, 6, 7, 8). The buried surface areas for the n425/NiVM G complex or the n425/NiVB G complex are 1056 Å2 or 1096 Å2, respectively. The overall structures of the two complexes are superimposable for the NiV G and the antibody variable domains, with a root-mean-square deviation (RMSD) of 0.82 Å. The n425/NiVM G complex shows very similar structure features to those of the n425/ NiVB G complex (Fig. 3a, b). The structure revealed that n425 CDRH3 and CDRH2/CDRH1 form a clamp-shaped conformation, clamping the NiV G rim region formed by the β1S3-β1S4 loop and the β6S4-β1S1 loop, with CDRH2 interacting with the β2S3-β2S4 loop. All CDRs of n425 contribute to antigen binding and involve 22 residues of NiV G and 16 residues of n425 (including 9 residues from CDRH3). There are a total of 9 pairs of hydrogen bonds (H-bonds) and 4 patches of hydrophobic interactions, and the H-bonds are relatively distributed among the CDRs, including 5 H-bonds for CDRH3, 1 for CDRH2 and 3 for CDRH1. Hydrophobic effects drive a portion of the antibody-antigen (Ab-Ag) binding, as observed mainly between the tip of CDRH3 and the β1 sheet cavity of NiV G (Fig. 3c, d). The n425 CDRH1 residue Y32 forms 3 H-bonds with the main chains of NiVB G D257, R258, and G259. Residue S56 of CDRH2 is surrounded by residue N325 form 1 H-bond. Residues L100, V103, W104, G105 and W109 on CDRH3 form five H-bonds with M267, T206, L265 and Y205 of NiVB G (Fig. 3d). With respect to hydrophobic interactions, residues W47 and L100 of n425 form a hydrophobic patch with V270 of NiVB G, and residues V103 and W104 form an intensive hydrophobic pocket with F229, Y231, L265, M267, R589 and L592 of NiVB G (Fig. 3d). In addition, among the 22 involved residues of NiVB G, 20 are strictly conserved across HeV, NiVM, and NiVB, and only 2 residues are conservatively substituted from NiV G to HeV G (Fig. 3e, f, Supplementary Fig. 9). We further explored whether the n425 binding epitope is conserved across any other henipavirus including Cedar virus, Ghana virus, Angavokely virus, Mojiang virus, and Langya virus. We performed sequence alignments of the RBP domain, and found that numerous differences within the binding epitopes (Supplementary Fig. 9). Furthermore, the ELISA results also indicated that n425 lacks the ability to recognize and bind the related henipavirus (Supplementary Fig. 10). Therefore, n425 can recognize both HeV G and NiV G (including both the NiVB and NiVM strains) because of the conservation of the sequences and structures of the epitopes in these strains.
a Ribbon diagram of the NiVM-G head domain in complex with n425. The NiVM-G head domain is colored royal blue, while n425 is represented in brown. b Ribbon diagram of the NiVB-G head domain in complex with n425. NiVB-G head domain is colored in dodger blue, while n425 is represented in magenta. c Enlarged view of the interface between the NiVM-G head domain and n425 with selected residues rendered as sticks. Dotted lines indicate the hydrogen bonds and dashed circles highlight the hydrophobic pockets. d Enlarged view of the interface between the NiVB-G head domain and n425 with selected residues rendered as sticks. Dotted lines indicate the hydrogen bonds and the hydrophobic pockets are highlighted by dashed circles. e Molecular surface representation of the NiV-G head domain with the n425 CDR loops. The three CDRs are shown as ribbons, and the NiV-G head domain is depicted in blue as a surface representation, highlighting the binding epitope in brown. f Molecular surface representation of the NiV-G head domain showing the n425 footprint colored by residue conservation among the NiV-G and HeV-G glycoproteins. Conservative sub: conservative substitution.
However, the binding epitope of n425 is distinct from that of ephrinB2-competing antibodies, such as m102.4 and HENV-26. Consistent with the results of the binding competition assay (Fig. 2a), n425 exhibited certain structural features similar to those of the antibody HENV-32 (Supplementary Fig. 11). Although all CDRs of both n425 and HENV-32 target the bottom side, which is usually hidden in the NiV G head domain, the two antibodies exhibit significantly different binding modes. In the HENV-32/HeV-RBP complex (PDB ID: 6VY4), most epitope residues that interact with HENV-32 are located within the N-terminal T196-I209 segment, and the β1S3/β1S4 loop13, whereas n425 not only engages these regions but also binds to the β2S3-β2S4 loop. This difference in binding modes between n425 and HENV-32 could be attributed to different conformations of their paratope areas and CDRH3s since the interface between Ab and Ag in the n425/NiV G complex is wider and more tightly bound than that found in the HENV-32/HeV-RBP complex (Supplementary Fig. 11a, b). In addition, n425 has a relatively long, protruding CDRH3 with a β-hairpin conformation that extends into a β1-sheet of NiV G (Supplementary Fig. 11a).
UdAb n425 disrupts cell fusion and NiV entry by targeting the G head domain interface
The epitope recognized by n425 is located in the putative dimeric interface of G head domains rather than binding to the ephrin receptor binding site. Consequently, we proposed that n425 inhibits viral entry into cells by interfering with its F fusion-triggering mechanism. To test this hypothesis, we established a NiVM G and F protein-mediated cell-cell fusion assay to determine whether NiV fusion is influenced by n425. Strikingly, n425 was found to be highly potent at inhibiting cell-cell fusion and syncytium formation, either as a monovalent single-domain antibody or as a bivalent Fc fusion protein, with IC50 values of 0.123 and 0.028 µg/ml, respectively (Fig. 4a). In contrast, the receptor-competing antibody m102.4 exhibited only moderate cell-cell fusion inhibition, with an IC50 (4.230 µg/ml) 151-fold higher than that of the bivalent antibody n425.
a n425 inhibits membrane fusion mediated by NiVM glycoproteins in a fusion inhibition assay. Left: Images of henipaviruses cell-cell fusion in the presence of 100 μg/mL n425, n425-Fc, or m102.4. Scale bars, 50 μm. Right: Analysis of the inhibitory activities of these antibodies against henipavirus-mediated cell-cell fusion. The mean ± S.D. from three biologically independent experiments is shown. Source data are provided as a Source Data File. b The cryo-EM structures of the NiVB-G(S76C) tetramer (dodger blue) with n425 (magenta). c Overlay of the NiVB-G monomer (dodger blue) with n425 (magenta) and the NiV-G ectodomain tetrameric structures (dark sea green) (PDB ID: 7TY0 and 7TXZ).
To further investigate the underlying mechanism of action of n425, we determined the structure of the NiV G ectodomain homotetramer complexed with n425 at a resolution of 5.87 Å using single-particle cryo-electron microscopy reconstruction (Supplementary Fig. 7, 8). Intriguingly, we noticed that four UdAbs respectively bound to the head domains of the NiV G tetramer, in which n425 binds to the side and locks the complex in a conformation with all four heads oriented upward (Fig. 4b). We next overlaid the n425/NiV G monomer structure with the nAH1.3/NiV G tetramer structure (PDB ID: 7TY0 and 7TXZ) and found that the combination of n425 in the middle of the stalk obviously affected the tetramerization of NiV G (Fig. 4c). Since n425 binds to the inner side of NiV G, it destabilizes G protein tetramerization and consequently impairs F protein activation, thereby hindering membrane fusion. These results demonstrated that disruption of G tetramerization could be a mechanism of action for the antibodies targeting interface epitopes.
Therapeutic efficacy of n425 in suckling mouse models
Our previous studies showed that UdAb displays markedly superior tissue permeability compared to that of conventional IgG antibodies, which could be attributed to its smaller size15,18. Since the brain is the primary site of NiV infection, we initially investigated the ability of UdAb n425 to penetrate the blood-brain barrier (BBB). The antibodies were labeled with 125I and administered through intravenous injection in BALB/c mice, followed by the collection of brain tissue samples for the quantification of antibodies (Fig. 5a). The results confirmed that n425 demonstrated more efficient penetration into the brain than m102.4 (Fig. 5b). To explore the therapeutic efficacy of n425, we established two murine animal models by intrathoracic or intracerebral administration of NiVM pseudoviruses into suckling mice19, representing NiV respiratory and brain infections, respectively (Fig. 5c). Two hours later, the mice were systemically administered m102.4 (150 kDa), UdAb n425 (14 kDa), or two larger variants of n425 (n425-sFc-n425, 55 kDa; n425-Fc, 85 kDa) (Supplementary Fig. 12). Interestingly, while all antibodies effectively cleared the pseudovirus in the thoracic region (Fig. 5d), the two smaller antibodies (n425 and n425-sFc-n425) demonstrated significantly stronger efficacy in eliminating pseudovirus in the brain than the two larger antibodies (n425-Fc and m102.4) (Fig. 5e). These data indicate the therapeutic potential of n425 for treating NiV infection, particularly in the context of NiV brain infection, which is a major contributor to high mortality.
a Schematic design of the biodistribution study in BALB/c mice. Mice (n = 12 mice/group) were intravenously injected via the tail with 5 mg/kg 125I-n425 or 125I-m102.4. Three mice were euthanized at each time point (0, 15, 30, and 60 min) for blood and brain collection. b The antibody concentration in the brain and blood was calculated as a percentage of the injected dose per gram of tissue mass (%ID/g). Data are represented as the mean ± S.D. c Schematic representation of treatment with four types of antibodies in the NiVM pseudovirus-infected mouse model. Suckling mice were intracerebral or intrathoracic (i.t.) inoculated with NiVM pseudoviruses and subsequently treated with PBS. On d 3, the mice were anesthetized for fluorescence examination. d, e Suckling mice were intracerebral or intrathoracic inoculated with NiVM pseudoviruses and subsequently treated with n425, n425-sFc-n425, n425-Fc, or mAb m102.4 via the intraperitoneal route at a dose of 5 mg/kg at 2 h post-challenge. On d 3, the bioluminescence signal for each mouse was detected using the IVIS-Lumina III imaging system. The data are expressed as the means ± S.D. (n = 6 mice per group for intrathoracic infectious model; n = 5 mice per group for intracerebral infectious model). Significant differences between the two independent groups were determined by the Mann‒Whitney U test using Prism software (*, p < 0.05; **, p < 0.01). The illustrations in (a, c) were created with BioRender.com under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license. Source data are provided as a Source Data File.
Discussion
To develop effective countermeasures, it is crucial to understand the mechanisms by which NiV-neutralizing antibodies interact with the virus, as well as their potential for cross-neutralization against these NiV strains. The UdAb n425 reported here binds to a novel epitope located on the head domain of the NiV G glycoprotein. This binding epitope is entirely distinct from the epitopes of reported antibodies, such as m102.4, HENV-26, hAH1.3, and nAH1.3. While they partially overlap with the epitope of HENV-32, significant differences can be observed in the orientation and mode of antibody binding. Moreover, in the binding epitope of n425, only two amino acids vary among HeV, NiVM, and NiVB isolates, fewer than the nonconserved amino acids found in the epitopes of other antibodies (m102.4, 5; HENV-26, 9; hAH.3, 8; nAH.3, 6; and HENV-32, 4). Therefore, the discovery of this conserved epitope may aid in the design of broad-spectrum vaccines against henipaviruses.
Interestingly, n425 binds to a cryptic epitope on the dimeric interface of the NiV G protein, resembling the recent discovery of hidden epitopes on the trimeric interface of hemagglutinin in influenza virus20,21,22 or the spike protein in SARS-CoV-215. Unique binding epitopes also result in distinct mechanisms of action for antibodies. For instance, the binding of antibodies to the conserved epitope on the spike trimeric interface induced the decay and disassembly of the SARS-CoV-2 spike trimer, resulting in potent viral neutralization16. Similarly, we found that n425 binding to the dimeric interface of the NiV G protein disrupted tetramerization of the G protein, thereby inhibiting membrane fusion. The restructuring of the quaternary structure resulting from n425 binding may render the activation sites in the stem areas of the NiV G unreachable by the fusion proteins, consequently inhibiting F protein activation. Indeed, n425 exhibits a potency two orders of magnitude greater than m102.4 in inhibiting membrane fusion. These findings have enriched our understanding of the mechanisms by which antibodies neutralize henipaviruses.
Notably, NiV infection occurs in the brain, leading to neurological symptoms, including highly lethal encephalitis in humans23. Furthermore, some patients experience neurologic relapse, even after an initially mild illness. Recently, NiV was found to persist in the brains of nonhuman primate survivors, indicating that late-onset and relapsing encephalitis in human survivors may result from NiV persistence in the brain24, posing significant challenges for biological drug treatments owing to their limited ability to penetrate the BBB25. Here, UdAb n425 serves as the smallest antigen-binding fragment that can be derived from a conventional antibody molecule. Indeed, recent studies have demonstrated the exceptional tissue penetrance of UdAb compared to that of conventional IgGs18. We also observed that n425 has significantly greater brain penetrance than m102.4. In the suckling mouse model, both n425 (15 kDa) and single-chain Fc-fused divalent n425 (55 kDa) exhibited superior therapeutic efficacy compared to larger antibodies (85, 150 kDa). Additionally, the single-chain Fc-fused n425 maintains a favorable in vivo pharmacokinetic profile based on preserved FcRn binding despite its smaller size26,27,28,29,30. Alternatively, the fusion of n425 with albumin-binding UdAbs also offers a potential strategy to prolong the half-life of UdAbs31. These findings underscore the unique advantages of n425 in the treatment of NiV brain infections. However, it should be noted that pseudovirus infection models were used instead of live virus models due to the lack of access to BSL-4 laboratories. Future studies will aim to confirm these findings using authentic virus infection models.
In summary, the findings presented herein shed light on the unique characteristics of UdAb n425 as a potent neutralizing antibody against NiV. By targeting a conserved epitope on the head domain of the NiV G glycoprotein, we found that it may disrupt G protein tetramerization, thereby hindering F protein activation and membrane fusion. These insights enrich our understanding of henipavirus neutralization mechanisms and open avenues for the design of effective countermeasures.
Methods
Ethics statements
Female BALB/c mice were purchased from the Shanghai Model Organisms Center and maintained under SPF conditions. All procedures related to animal handling, care, and treatment were performed and approved by the Ethics Committee of the School of Basic Medical Sciences at Fudan University (Ethical approval number: 20210302-110) in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of Fudan University.
Cells and viruses
Vero E6 cells (catalog no. CRL-1586), and 293 T cells (catalog no. CRL-3216) were obtained from the American Type Culture Collection (ATCC). U87 cells (catalog no. TCHu138) were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Meilunbio) supplemented with 10% fetal bovine serum (FBS, Gibco) at 37 °C in a 5% CO2 atmosphere. Expi293F cells (catalog no. A14527) were obtained from Thermo Fisher and maintained in Expi293F expression medium (Thermo Fisher) at 37 °C in a 5% CO2 atmosphere.
Expression and purification of the Henipavirus attachment glycoprotein
Sequence-optimized DNA fragments encoding the G protein head domain from HeV (residues 185-604), NiV-Malaysia (residues 183-602), NiV-Bangladesh (residues 185-602), Cedar virus (residues 209-622), Mojiang virus (residues 166-652), and Ghana virus (residues 199-632) were synthesized (Genscript), cloned, and inserted into the pSecTag2B vector incorporating an IgG1 Fc region at the C-terminus to facilitate protein purification. For the complex structure of n425 and NiV-G, the full-length ectodomain of G protein, containing an N-terminal stalk, a neck domain, a linker region, and a C-terminal head domain was used. The encoding fragments (residues 71-602) were synthesized and cloned into the pcDNA3.1( + )-C-6His vector. To stablize tetramers of the G ectodomain, the amino acid serine at position 76 was mutated to cysteine32 by a site-directed mutagenesis kit (Vazyme), and subsequently, an interchain disulfide bond was formed. The recombinant plasmids were verified by sequencing and then transiently transfected into Expi293F cells using EZ-Trans (Life-iLab). The culture supernatants were harvested after 3 d. The G protein was purified by Ni-NTA (Yeasen), and the head domain of the G protein was purified by Protein A resin (GenScript) following the manufacturer’s instructions. A further size-exclusion chromatography (SEC) polishing purification step was performed on a Superdex 200 Increase 10/300 column (GE Healthcare) by the AKTA pure system to separate monomers or tetramers from contaminants, as well as buffer exchange of the protein into PBS buffer (HyClone). Protein integrity was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS‒PAGE).
Expression and purification of recombinant IgG
Synthetic heavy- and light-chain variable region genes for previously reported neutralizing antibodies against Henipavirus, including HENV-26, HENV-32, m102.4, nAH1.3, and hAH1.3, were subcloned and inserted into a pTT expression vector in the human antibody IgG1 format. IgGs were produced by transient transfection of Expi293F cells as described above. Five days posttransfection, the supernatants were harvested, clarified by low-speed centrifugation, and incubated overnight with Protein G resin (GenScript). The resin was collected on a chromatography column, washed with PBS (pH 7.4), and eluted in 0.1 M glycine (pH 2.7). The eluates were equilibrated using 1 M Tris-HCl (pH 9.0), and the buffer was immediately replaced with PBS (pH 7.4).
Generation of human single-domain antibodies against the Henipavirus G protein from a phage library
Henipavirus G proteins were biotinylated via nonspecific labeling using sulfo-NHS-LC-Biotin (Thermo Fisher). Library phages were preblocked in 3% milk powder (w/v) in PBS (MPBS) and incubated with biotinylated G protein and streptavidin-coated magnetic beads (Thermo Fisher). After the phages were washed with PBST (PBS supplemented with 0.05% Tween 20), G-bound phages were used to infect mid-log phase TG1 bacteria (Lucigen), and 2 h later, M13KO7 helper phages (Thermo Fisher) were added to rescue the phagemids. After incubation overnight, the phages in the culture supernatant were precipitated using PEG8000-NaCl and resuspended in PBS for subsequent panning. The enrichment of antigen-specific phages after each round of panning was assessed by a polyclonal phage enzyme-linked immunosorbent assay. Positive clones expressing human single-domain antibodies were identified from the enriched rounds of panning by phage-based enzyme-linked immunosorbent assay20.
Production of human single-domain antibodies
The sequences of the identified clones were subcloned and inserted into the pComb3x vector downstream of the OmpA signal peptide (MKKTAIAIAVALAGFATVAQA). A His-tag and a Flag-tag in tandem were also incorporated at the C-terminus. Human single-domain antibody expression was performed in Escherichia coli HB2151 bacterial culture at 30 °C for 14 h in the presence of 1 mM IPTG. E. coli cells were harvested, resuspended in histidine-binding buffer, and lysed with polymyxin B (Sigma‒Aldrich). The antibodies in the supernatant were purified by Ni-NTA according to the manufacturer’s manual (Yeasen) and further concentrated using an Amicon ultracentrifugal concentrator (Millipore) with a molecular weight cutoff of 3 kDa.
The n425-sFc-n425 protein used in this study was constructed by direct linkage of engineered Fcs between the n425 sequences. An engineered Fc is a kind of single-chain Fc (sFc) generated by mutating critical residues located on the IgG1 Fc dimerization interface26. The coding sequence described above was first amplified by PCR and then incorporated into the pSecTag2B vector via the SfiI and NotI restriction sites to remove the C-terminal Fc tag. The n425-Fc protein consisted of the n425 coding sequence fused to the pSecTag2B vector, followed by the incorporation of an IgG1 Fc region at the C-terminus. Proteins were expressed using the Expi293F Expression System and purified with Protein G resin (GenScript).
Comparison of the half maximal effective concentration (EC50) for binding
To determine the binding ability of the human single-domain antibody to HeV-G, NiVM-G, NiVB-G, Cedar virus-G, Mojiang virus-G and Ghana virus-G, the half maximal effective concentration (EC50) values were measured by ELISA using wells of a 96-well plate that were coated overnight at 4 °C with 100 ng/well soluble protein. The wells were blocked for 1 h with 3% MPBS at 37 °C. After the wells were washed with PBST, purified antibodies, starting at 15 μg/ml in threefold serial dilutions, were added, and the plates were incubated for 1.5 h at 37 °C. A monoclonal anti-Flag-HRP antibody (Sigma‒Aldrich, cat#A8592, clone no. M2) diluted at 1:10,000 in blocking solution was added to each well for a 1 h incubation. The wells were washed 5 times with PBST, the substrate ABTS (Invitrogen) was subsequently added to each well, and signal reading was carried out at 405 nm after incubation for 10 min using a microplate spectrophotometer (BioTek). EC50 values were determined by sigmoidal dose-response nonlinear curve fitting with Prism software (GraphPad).
Antibody affinity determination by biolayer interferometry
The kinetics of the binding of the single-domain antibody to the G protein were determined on an OctetRED96 device (Sartorius). Purified NiVM G protein at 20 μg/ml buffered in sodium acetate (pH 5.0) was immobilized onto activated AR2G biosensors (Sartorius) and then incubated with threefold serial dilutions of 100 nM n425 in kinetics buffer for 600 s after a short baseline step. The sensors were transferred into a kinetics buffer to allow a 600 s dissociation step. Data analysis software was used for curve-fitting with a 1:1 binding model to calculate equilibrium dissociation constant values.
Epitope-binning analysis for single-domain antibodies
To determine whether the single-domain antibody n425 binds to the same epitope as other G-specific antibodies and blocks host receptor ephrinB2 binding, a competition binding assay using BLI was performed17. Recombinant HeV or NiV G protein was covalently coupled to the activated AR2G sensor surface. The loaded biosensors were transferred into wells containing the single-domain antibody n425 at a concentration of 100 nM and incubated until binding saturation, followed by incubation with the secondary reference antibodies HENV-26, HENV-32, m102.4, nAH1.3, hAH1.3, or soluble ephrinB2 (Sino Biological) in the presence of n425 for 600 s. The ratio of the maximal signal from the secondary antibody after the first antibody bound to the maximal signal of the secondary antibody tested alone was calculated and expressed as a percentage. The data were analyzed using Data Analysis software, and the ratio of the maximal binding signal from the secondary antibody, or ephrinB2, after n425 saturated binding to that of the secondary antibody, or ephrinB2, associated alone, was calculated and expressed as percentage binding.
Establishment and validation of a panel of Henipavirus pseudoviruses
We used the vesicular stomatitis virus (VSV) system to construct pseudovirus models of Henipavirus. The VSV-G-rLuc provided by our collaborators is a pseudovirus particle that packages expression cassettes for renilla luciferase instead of the VSV enveloped G protein encoding gene in the VSV genome. Therefore, it is necessary to first package the VSV-G backbone containing the VSV-G protein gene for the establishment of the Henipavirus pseudovirus system. Briefly, 2×106 293 T cells were seeded into 10 cm cell culture plates in 10 ml of DMEM supplemented with 10% FBS and incubated at 37 °C and 5 % CO2 for 16 h. The plasmid pMD2. G (encoding the VSV enveloped G protein) (24 µg) was transfected into 293 T cells using 10 µl VigoFect transfection reagent (Vigorous Biotechnology), and the cells were incubated at 37 °C and 5% CO2 for 6 h. After transfection, the VSV-G-rLuc pseudovirus particles were added to the cells and incubated for 2 h. The culture medium was replaced with fresh DMEM supplemented with 10% FBS. After 72 h, the supernatant containing the luciferase reporter-expressing VSV-G backbone pseudoviruses was harvested by centrifugation at 3000 ×g for 10 min and filtered through a 0.45 µm pore-size filter before being stored at -80 °C in aliquots until use.
Genes encoding the G or F protein from Henipavirus HeV, NiVM, and NiVB were synthesized (GenScript) and then subcloned and inserted into the pcDNA3.1 vector. The recombinant plasmids encoding F protein and G protein were confirmed by DNA sequencing and mixed at a ratio of 1:1, followed by a transfection step in 293 T cells at a density of 1.5 × 107. After 6 h, the luciferase reporter-expressing VSV-G backbone was added to the cells and were cultured for 2 h, and the culture medium was replaced with fresh DMEM supplemented with 10% FBS. After 72 h, the supernatant containing the pseudoviruses was harvested by centrifugation at 3000 × g for 10 min and filtered through a 0.45 µm pore-size filter. The filtered supernatant was concentrated by centrifugation at 100,000 × g for 3 h before being stored at –80 °C in aliquots until use.
The Vero E6 cells were used to determine the Henipavirus pseudoviruses titration. In brief, tenfold serially diluted pseudoviruses were used to infect Vero E6 cells for 12 h. The supernatant was refreshed 12 h postinfection, and the cells were cultured for an additional 48 h. Luciferase activity was recorded to determine the infectivity of the pseudoviruses and the median tissue culture infectious dose (TCID50) was calculated by Reed–Muench Method.
Pseudovirus neutralization assay
Vero E6 cells at a density of 10,000 per well were coated in the wells of a 96-well cell culture plate overnight to form a monolayer of adherent cells. According to the results of the pseudoviral infectivity assay mentioned above, a relative light unit (RLU) was used for viral dilutions of approximately 20,000. A threefold serially diluted antibody alone or neutralizing antibody cocktail were incubated with pseudovirus at a 1:1 ratio for 1 h at 37 °C. The starting concentration of the antibody was 100 μg/ml. In the case of the antibody cocktail, each of the two antibodies in the cocktail was present at half of the indicated concentrations. The antibody/pseudovirus mixtures were added and incubated with U87 cells for 12 h, after which the culture medium was replaced with fresh DMEM supplemented with 10% FBS. The cells were incubated for another 48 h, washed with PBS twice, and subsequently lysed with 50 µl of lysis reagent (Promega). Thirty microliters of cell lysates were added to the substrate of a Firefly Luciferase Assay Kit (Promega), and the RLU readout was detected. The percent inhibition was calculated as the relative reduction in RLU compared with that of the control well (cells and pseudovirus added without antibody). The data were nonlinearly fitted, and the half-maximal inhibitory concentrations (IC50) were calculated via regression of the four parameters using GraphPad Prism. The corresponding combination index (CI) values were calculated using CompuSyn software and determined by comparing the IC50 of the antibody alone to that of the antibody cocktail.
Construction of cell-cell fusion models and cell-cell fusion inhibition
To construct a visiable cell fusion system, gene encoding the F protein from Henipavirus was cloned to the pAAV-IRSE-EGFP vector. 2 × 106 293 T cells were seeded into 10 cm cell culture plates in 10 ml of DMEM supplemented with 10% FBS and incubated at 37 °C and 5 % CO2 for 16 h. The pAAV-IRSE-NiVM F-EGFP and pcDNA3.1-NiVM G (10 µg) plasmids were transfected into 293 T cells using the transfection reagent (10 µl) VigoFect (Vigorous Biotechnology). After 48 h of transfection, the effector cells (293 T) were dissociated using cell dissociation solution (Gibco) and incubated (2×104 cells/well, 60 µl) with or without antibodies at 37 °C for 1 h. All antibodies (n425, n425-Fc, and IgG m102.4) were threefold serially diluted from 90 μg/ml to 0.01 μg/ml in a 96-well plate. The target Vero E6 cells (receptor ephrin-B2 on the cell surface) were seeded separately at 7.5×105 per well in a six-well dish in 3 ml of DMEM supplemented with 10% FBS and incubated overnight at 37 °C and 5 % CO2 to form a monolayer of adherent cells. A mixture of effector cells and antibodies (100 µl) was then added to Vero E6 cells for 4-8 h at 37 °C and 5 % CO2. Cell fusion images were captured using fluorescence microscopy, and the number of syncretic cells within at least 5 randomly selected fields was counted. The fused cells were much larger and had weaker fluorescence intensity than the unfused cells because of the diffusion of EGFP from one cell to more cells.
Antibody footprint analysis
The structures of the complexes of the NiV G glycoprotein (PDB ID: 3D11) with HENV-26 (PDB ID: 6VY6), HENV-32 (PDB ID: 6VY4), m102.4 (PDB ID: 6CMG), hAH1.3 (PDB ID: 7SYY), and nAH1.3 (PDB ID: 7TXZ) were used to analyze the epitope footprints of previously described antibodies. Interface residues were identified by ePISA (http://www.ebi.ac.uk/pdbe/prot_int/pistart.html) using default parameters and checked in COOT33. The graphs were drawn using PyMOL34 or ChimeraX35.
Cryo-EM data collection and image processing
The purified NiV-G ectodomain was incubated with the human single-domain antibody n425 at a 1:2 molar ratio of excess n425 for 1 h at room temperature. The fractions containing the complex were eluted using a Superde ×200 Increase 10/300 column (GE Healthcare) and equilibrated in buffer containing 20 mM Tris and 200 mM NaCl at pH 8.0, followed by a quality control step by negative-staining EM. The NiV-G/n425 complex was concentrated to 0.5 mg/ml using a Vivaspin centrifugal filter device (100 kDa MW, Sartorius). The sample (3 μl) was applied to a freshly glow-discharged NiTi-Au 300 mesh R1.2/1.3 grid (Quantifoil) in a Vitrobot Mark IV (Thermo Fisher Scientific) at 4 °C, requiring a waiting time of 10 s and a blotting time of 2 s under 100% humidity conditions.
Cryo-EM data were captured on a Titan Krios G4 transmission electron microscope operated at 300 kV and equipped with a Falcon 4i camera and a Seletrics X Imaging Filter (Thermo Fisher) set to a slit width of 20 eV. Movie stacks were automatically collected in AFIS mode using EPU software at a nominal magnification of 130,000×, corresponding to a physical pixel size of 0.932 Å at a defocus range from –1.0 μm to –3.0 μm. The total dose of each EER movie stack was 50e/Å2, and the stack was dose-fractionated to 1080 frames. Movie stacks were imported to RELION 3.136 and then motion-corrected (binned by 2 and dose-weighted) by MotionCor237, and the contrast transfer function (CTF) was estimated by Gctf38.
In total, 16,770 selected good images of NiVB-G(S76C)/n425 were imported to cryoSPARC39 for patch CTF estimation, blob picking, and 2D classification. Good classes were further used as templates for template picking. Both tetramers and monomers, as well as dimers and trimers, were observed. A total of 526,396 good monomer particles were imported to RELION for 3D classification and autorefinement, yielding a map at 3.22 Å resolution with 434,195 particles. A total of 1,306,559 tetramer particles were imported to RELION for multiple rounds of 3D classification and exported back to cryoSPARC for NU-Refine, yielding a map at 5.87 Å resolution. In total, 4,251 NiVM_n425 micrographs were selected, and a similar procedure was used, yielding a 3.63 Å monomer map with 661,646 particles. The resolutions were estimated using the Gold-standard Fourier shell correlation (FSC) at the 0.143 criterion. Maps were sharpened using EMReady40, and handedness was corrected using UCSF Chimera41.
Model building and refinement
The NiV-G_nAH1.3 model (PDB ID: 7TXZ) was used as the initial model of NiVB and NiVM; the initial model of n425 was generated using the Swiss model42 for structure prediction. Initial models were fit to maps using UCSF Chimera, and further model adjustment was performed in COOT33; real-space refinement was performed in PHENIX43. Model validation was performed using Molprobity44. The figures were prepared using UCSF Chimera and UCSF ChimeraX45. The statistics of the model refinement and data collection are listed in Table S1.
Preparation of 125I-labeling antibody and brain biodistribution in vivo
Antibodies against n425 and IgG1 m102.4 were labeled with 125I using the Iodogen method46. Briefly, the concentration of the antibody solution was adjusted to 2 mg/ml in 50 mM PBS (pH 7.4). Subsequently, 50 μl of 2 mg/ml antibodies and 10 μl of Na125I solution (approximately 45 MBq) were added to a vial precoated with 50 μg of iodogen and mixed gently. The mixture was then incubated at room temperature for 10 min and purified by a PD-10 desalting column. The radioactive fractions containing the 125I antibody were collected and filtered through a 0.2 μm vacuum filter for further in vivo experiments. The labeling quality was determined by silica gel paper chromatography, and 85% methanol-water was used as the developing solution.
Six- to eight-week-old female BALB/c mice were used to perform a biodistribution study and were randomly divided into two groups (n = 12/group). After intravenous administration of a single dose of 5 mg/kg 125I-n425 or 125I-m102.4, three mice were euthanized at each time point (0, 15, 30, and 60 min), and the blood and brain were collected. Blood samples were centrifuged for 3 min at 3,000×g, and the plasma was separated and stored at –80 °C. Brain tissues were weighed and homogenized to collect the supernatant. The radioactivity of these samples was measured with an automated gamma counter. The brain uptake was calculated as a percentage of the injected dose per gram of tissue mass (%ID/g).
Therapeutic efficacy in mice with NiVM pseudovirus
Female BALB/c suckling mice were inoculated via the intracerebral or intrathoracic route with 107 NiVM pseudoviruses under anesthesia on D0. Two hours later, the challenged mice were randomly divided into five groups (n = 5-6/group) and treated with n425, n425-sFc-n425, n425-Fc, or mAb m102.4 via the intraperitoneal (i.p.) route at a dose of 5 mg/kg. The control group was subjected to PBS treatment. On D3, the mice were anesthetized for fluorescence examination. After administering D-luciferin, the bioluminescence signal was detected for each mouse with an acquisition time of 1 min using the IVIS-Lumina III imaging system (PerkinElmer). The relative bioluminescence was calculated using the photon-per-second mode with normalization for the imaging area (photons/sec/cm2/sr).
Statistical analysis
The results of antibody binding ability to G protein by ELISA, virus neutralization, cell-cell fusion inhibition, and in vivo data are presented as the mean value ± standard deviation (S.D.). All the data were analyzed using Prism software (GraphPad Software). Significant differences between the two independent groups in vivo were determined by the Mann‒Whitney U test. P values < 0.05 were considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The cryo-EM maps and coordinates of n425 in complex with NiVM-G monomer have been deposited in the Electron Microscopy Data Bank (EMDB) and Protein Data Bank (PDB) with accession numbers EMD-38563 and PDB 8XPY. The cryo-EM structures of n425 in complex with NiVB-G(S76C) monomer have been deposited with accession numbers EMD-38560 and PDB 8XPS. The accession numbers of n425-NiVB-G(S76C) tetramer are EMD-38564 and PDB 8XQ3. This study also used 6CMG, 6VY6, 6VY4, 7SYY, 7TXZ, and 7TY0 from the Protein Data Bank. All data associated with this study are presented in the paper or the Supplementary Information. Source data are provided with this paper.
References
Eaton, B. T., Broder, C. C., Middleton, D. & Wang, L. F. Hendra and Nipah viruses: Different and dangerous. Nat. Rev. Microbiol 4, 23–35 (2006).
Chua, K. B. et al. Nipah virus: a recently emergent deadly paramyxovirus. Science 288, 1432–1435 (2000).
Lee, B., Broder, C. Christopher., Wang, Lin-Fa. in Fields Virology: Emerging viruses Ch. 13, (Lippincott Williams & Wilkins, 2020).
Wang, Z. et al. Potent monoclonal antibody-mediated neutralization of a divergent Hendra virus variant. Proc. Natl. Acad. Sci. USA 119, e2122769119 (2022).
Charlier, J. et al. Disease control tools to secure animal and public health in a densely populated world. Lancet Planet Health 6, e812–e824 (2022).
Zhu, Z. et al. Potent neutralization of Hendra and Nipah viruses by human monoclonal antibodies. J. Virol. 80, 891–899 (2006).
Bossart, K. N. et al. A neutralizing human monoclonal antibody protects african green monkeys from hendra virus challenge. Sci. Transl. Med. 3, 105ra103 (2011).
Geisbert, T. W. et al. Therapeutic treatment of Nipah virus infection in nonhuman primates with a neutralizing human monoclonal antibody. Sci. Transl. Med. 6, 242ra282 (2014).
Bossart, K. N. et al. A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute nipah virus infection. PLoS Pathog. 5, e1000642 (2009).
Zhu, Z. et al. Exceptionally potent cross-reactive neutralization of Nipah and Hendra viruses by a human monoclonal antibody. J. Infect. Dis. 197, 846–853 (2008).
Playford, E. G. et al. Safety, tolerability, pharmacokinetics, and immunogenicity of a human monoclonal antibody targeting the G glycoprotein of henipaviruses in healthy adults: a first-in-human, randomised, controlled, phase 1 study. Lancet Infect. Dis. 20, 445–454 (2020).
Wang, Z. et al. Architecture and antigenicity of the Nipah virus attachment glycoprotein. Science 375, 1373–1378 (2022).
Dong, J. et al. Potent henipavirus neutralization by antibodies recognizing diverse sites on Hendra and Nipah virus receptor binding protein. Cell 183, 1536–1550.e1517 (2020).
Xu, K. et al. Crystal structure of the Hendra virus attachment G glycoprotein bound to a potent cross-reactive neutralizing human monoclonal antibody. PLoS Pathog. 9, e1003684 (2013).
Li, C. et al. Broad neutralization of SARS-CoV-2 variants by an inhalable bispecific single-domain antibody. Cell 185, 1389–1401 e1318 (2022).
Hao, A. et al. Defining a highly conserved cryptic epitope for antibody recognition of SARS-CoV-2 variants. Signal Transduct. Target Ther. 8, 269 (2023).
Wu, Y. et al. Identification of Human Single-Domain Antibodies against SARS-CoV-2. Cell Host Microbe 27, 891–898 e895 (2020).
Wu, Y. et al. A highly stable human single-domain antibody-drug conjugate exhibits superior penetration and treatment of solid tumors. Mol. Ther. 30, 2785–2799 (2022).
Nie, J. et al. Nipah pseudovirus system enables evaluation of vaccines in vitro and in vivo using non-BSL-4 facilities. Emerg. Microbes Infect. 8, 272–281 (2019).
Yu, F. et al. A potent germline-like human monoclonal antibody targets a pH-sensitive epitope on H7N9 influenza hemagglutinin. Cell Host Microbe 22, 471–483 e475 (2017).
Bangaru, S. et al. A site of vulnerability on the influenza virus hemagglutinin head domain trimer interface. Cell 177, 1136–1152 e1118 (2019).
Watanabe, A. et al. Antibodies to a conserved influenza head interface epitope protect by an IgG subtype-dependent mechanism. Cell 177, 1124–1135.e1116 (2019).
Goh, K. J. et al. Clinical features of Nipah virus encephalitis among pig farmers in Malaysia. N. Engl. J. Med. 342, 1229–1235 (2000).
Liu, J. et al. Nipah virus persists in the brains of nonhuman primate survivors. JCI Insight 4, https://doi.org/10.1172/jci.insight.129629 (2019).
Terstappen, G. C., Meyer, A. H., Bell, R. D. & Zhang, W. Strategies for delivering therapeutics across the blood-brain barrier. Nat. Rev. Drug Discov. 20, 362–383 (2021).
Wang, C. et al. Engineered soluble monomeric IgG1 Fc with significantly decreased non-specific binding. Front Immunol. 8, 1545 (2017).
Ying, T., Gong, R., Ju, T. W., Prabakaran, P. & Dimitrov, D. S. Engineered Fc based antibody domains and fragments as novel scaffolds. Biochim Biophys. Acta. 1844, 1977–1982 (2014).
Ying, T., Ju, T. W., Wang, Y., Prabakaran, P. & Dimitrov, D. S. Interactions of IgG1 CH2 and CH3 Domains with FcRn. Front Immunol. 5, 146 (2014).
Pyzik, M., Kozicky, L. K., Gandhi, A. K. & Blumberg, R. S. The therapeutic age of the neonatal Fc receptor. Nat. Rev. Immunol. 23, 415–432 (2023).
Wu, H. F. et al. Half-life extension enhances drug efficacy in adeno-associated virus delivered gene therapy. Eng. Prc. 21, 203–213 (2023).
Sleep, D. Albumin and its application in drug delivery. Expert Opin. Drug Deliv. 12, 793–812 (2015).
Ortega, V. et al. Novel roles of the Nipah virus attachment glycoprotein and its mobility in early and late membrane fusion steps. mBio 13, e0322221 (2022).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr D. Biol. Crystallogr 66, 486–501 (2010).
Rigsby, R. E. & Parker, A. B. Using the PyMOL application to reinforce visual understanding of protein structure. Biochem Mol. Biol. Educ. 44, 433–437 (2016).
Pettersen, E. F. et al. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. Elife 7, https://doi.org/10.7554/eLife.42166 (2018).
Zheng, S. Q. et al. MotionCor2: Anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zhang, K. Gctf: Real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: Algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Sanchez-Garcia, R. et al. DeepEMhancer: a deep learning solution for cryo-EM volume post-processing. Commun. Biol. 4, 874 (2021).
Pettersen, E. F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Waterhouse, A. et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 46, W296–W303 (2018).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr D. Struct. Biol. 74, 531–544 (2018).
Williams, C. J. et al. MolProbity: More and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018).
Goddard, T. D. et al. UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Gustavsson, T., Syvanen, S., O’Callaghan, P. & Sehlin, D. SPECT imaging of distribution and retention of a brain-penetrating bispecific amyloid-beta antibody in a mouse model of Alzheimer’s disease. Transl. Neurodegener. 9, 37 (2020).
Acknowledgements
This work was supported by grants from the Ministry of Science and Technology of China (2019YFA0904400 to T.Y., 2021YFC2302500 to L.S.), Shanghai Municipal Science and Technology Major Project (ZD2021CY001 to L.S.), National Natural Science Foundation of China (32270984 to Y.W.), Science and Technology Commission of Shanghai Municipality (23XD1400800 to T.Y.), Shanghai Municipal Health Commission (GWVI-11.2-YQ46 to Y.W.), Advanced Customer Cultivation Project of Wuhan National Biosafety Laboratory, Chinese Academy of Sciences (2022ACCP-MS06 to T.Y.) and R&D Program of Guangzhou Laboratory (SRPG22-003 to L.S.). We thank the Center of Cryo-Electron Microscopy, Fudan University, for support with cryo-EM data collection. We thank Yao Wang and Dexin Xu from the Key Laboratory of Medical Molecular Virology, Shanghai Frontiers Science Center of Pathogenic Microorganisms and Infection, School of Basic Medical Sciences, Shanghai Medical College, Fudan University, for technical support.
Author information
Authors and Affiliations
Contributions
Y. Wu, T.Y., L.S., L.L., F.W., S.J., and D.S.D. conceived and designed the study. Yulu Wang, Y.S., and Z.S. performed most of the experiments with assistance from C.W., J.Q., Q.M., Yajie Wang, W.S., Y.K., C.Z., Z.C., and Z.Y. T.Y., Y. Wu, Y.S. and L.S. integrated the data and wrote the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
Yulu Wang, Y. Wu, and T.Y. are listed as inventors on one patent application related to this work. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Zhaoqian Wang and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Y., Sun, Y., Shen, Z. et al. Fully human single-domain antibody targeting a highly conserved cryptic epitope on the Nipah virus G protein. Nat Commun 15, 6892 (2024). https://doi.org/10.1038/s41467-024-51066-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-51066-6