Abstract
The morbidity of hypertension is increasing among young adults worldwide, and glucose-6-phosphate dehydrogenase (G6PD) deficiency is a high-prevalence genetic disease. We investigated whether G6PD deficiency was associated with abnormal blood pressure (including elevated blood pressure and hypertension) among prepregnant reproductive-age females. We conducted a cross-sectional study in Shenzhen, which included 154 917 females aged 20–49 who participated in the National Free Pre-conception Check-up Projects supported by the Chinese government. After adjusting for confounding factors, the odds ratios (ORs) for the effects of G6PD deficiency on elevated blood pressure and hypertension were 1.18 (95% confidence interval (CI): 1.03–1.35) and 1.11 (95% CI: 1.00–1.23), respectively. Moreover, the association between G6PD deficiency and abnormal blood pressure was statistically significant for systolic blood pressure (SBP) but not significant for diastolic blood pressure (DBP). The multivariable-adjusted ORs for females with G6PD deficiency in the SBP 120–139 mm Hg and SBP ≥ 140 mm Hg groups were 1.10 (95% CI: 1.00–1.21) and 1.75 (95% CI: 1.25–2.42), respectively, while the multivariable-adjusted ORs for females with G6PD deficiency in the DBP 80–89 mm Hg and DBP ≥ 90 mm Hg groups were 1.09 (95% CI: 0.98–1.21) and 0.89 (95% CI: 0.66–1.19), respectively. Subgroup analyses showed similar results. The findings of this study underscored that reproductive-age females with a G6PD deficiency had a higher risk of elevated blood pressure and hypertension. Therefore, females with G6PD deficiency combined with elevated blood pressure or hypertension were high-risk populations during prepregnancy and pregnancy periods. Early intervention and collaborative management approaches should be explored to reduce the burden of these two diseases and improve maternal and child health.
Similar content being viewed by others
Introduction
Hypertension is a serious public health challenge worldwide due to its high prevalence and the associated concomitant risks of cardiovascular disease. It contributes to half of coronary heart disease and approximately 2/3 of cerebrovascular disease burdens [1]. In recent years, the morbidity of hypertension is increasing among young adults around the world [2, 3]. To emphasize the importance of early interventions for hypertension and to prevent more hypertension-related complications, the American Heart Association (AHA) developed a new definition for hypertension as ≥130/80 mm Hg in 2017. Additionally, the definition of “prehypertension” in the criteria from the seventh report of the Joint National Committee (JNC7) [4] was removed, which was divided into “elevated blood pressure” and “stage I hypertension” [5]. Furthermore, we observed that females with elevated blood pressure could be more likely to have hypertensive disorders during pregnancy [6], which is one of the main causes of maternal and perinatal morbidity and mortality [7, 8].
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is a high-prevalence X-linked genetic disease representing a low level of G6PD (an enzyme that catalyzes the first reaction in the pentose phosphate pathway) enzyme activity in cells [9, 10], and its prevalence is higher in males than in females [11, 12]. Reports indicate that more than 400 million people are affected worldwide [9]. Annual Chinese maternal and child health monitoring reports revealed that birth defects due to G6PD deficiency ranked third highest among birth defects in 2014 and 2015 [13, 14]. It has been suggested that G6PD deficiency is associated with reduced nicotinamide adenine dinucleotide phosphate (NADPH), which would result in vulnerability to oxidative damage [9, 15]. This oxidative damage status caused by downregulated G6PD is predisposes individuals to a variety of degenerative disorders such as cancer, diabetes, and cardiovascular diseases [16,17,18]. Previous studies have shown that “the oxidant stress–autoimmunity–inflammation interaction” is one of the most influential risk factors that causes hypertension [19,20,21]. Therefore, relevant studies on the association between G6PD deficiency and blood pressure are a concern among some scholars. An early study showed that G6PD-deficient individuals were predisposed to have elevated blood pressure [22], but another study observed that blood pressure among G6PD-normal and -deficient individuals was unchanged for systolic and diastolic blood pressure [23]. However, whether G6PD deficiency is associated with elevated blood pressure remains unclear.
Shenzhen is a city with >11 million people [24], with an average age of nearly 30 [25]. The city is located on the southern coast of China where the prevalence of G6PD deficiency in the female population is approximately 1.14–3.68% [26, 27]. Therefore, we performed a cross-sectional study to investigate the association between G6PD deficiency and abnormal blood pressure in Shenzhen among prepregnant reproductive-age females who participated in the National Free Pre-conception Check-up Projects (NFPCP), which is a national health service supported by the Chinese government [28].
The primary objective of this study was to determine whether G6PD deficiency is associated with elevated blood pressure and hypertension. The secondary objective was to explore the association of G6PD deficiency with systolic blood pressure (SBP) and diastolic blood pressure (DBP).
Methods
Participants and study design
The NFPCP is a nationwide, population-based cross-sectional study project aimed at providing free prepregnancy medical examinations for reproductive-age couples who have a pregnancy plan within 6 months [29]. The overall goal of the project is to reduce the incidence of adverse pregnancy outcomes throughout the country. Trained local health professionals use a questionnaire-based survey to complete a standardized family health file for each participating couple, as well as complete medical examinations. Completed files are then converted into electronic records and transferred into the NFPCP medical service information system for storage. This information system was developed by the National Research Institute for Family Planning and built with logic checks and instrument interfaces to avoid human errors. Prior to data submission, data workers recheck the data thoroughly.
All medical organizations in Shenzhen have included the G6PD enzyme activity screening program from the NFPCP since 2013. Activities at all study sites were implemented with standard operating procedures to ensure the reliability of the serology examination and to achieve excellent quality in the annual national external quality assessment. The detailed design and organization of this project are described elsewhere [30].
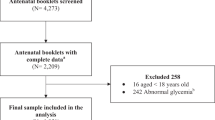
This study was an original research based on the NFPCP and data from 22 sites were obtained from the NFPCP system in Shenzhen, China. From 01 January 2013 to 31 December 2016, 180 389 eligible females aged 20–49 participated in the NFPCP. Participants with missing data for G6PD enzyme activity and blood pressure were excluded. Finally, data on 154 917 participants were included in the analysis (detailed information on the study population is shown in Fig. 1).
Data collection
The standardized questionnaires were completed through face-to-face interviews, while medical examinations were conducted by trained health professionals. Demographic characteristics and lifestyle information were collected by questionnaires from husbands and wives separately. The variables included the participant’s date of birth, nationality, urban/rural inhabitants, southern/northern residents based on the address of household registration, educational level, alcohol consumption, and husband’s active smoking status. Body weight and height were measured with light indoor clothes without shoes and other accessories based on a standardized protocol. Experienced physicians conducted blood pressure measurements from the participants’ right arms using an automatic sphygmomanometer after at least 10 min of rest following the NFPCP’s standard protocol. Erythrocyte G6PD enzyme activity, serum glucose and creatinine were tested using blood samples collected after at least 8 h of fasting using ethylenediamine tetra-acetic acid and heparin lithium-anticoagulant vacuum tubes. The samples were stored at 4 ℃ in the freezer and analyzed within 24 h. G6PD enzyme activity was tested using a standardized quantitative spectrophotometric analysis.
This study was conducted under the Declaration of Helsinki (2000) of the World Medical Association and approved by the Institutional Research Review Board at the National Population and Family Planning Commission. All participants provided written informed consent prior to their participation.
Variable definition
Variables in this study included demographic characteristics, lifestyle information and some serological test results. Age was split into three groups (20–29, 30–39, and 40–49), while nationality was categorized as ‘Han’ and ‘others’. We used high school education to categorize the educational level into two groups (high school or above and middle school or below). Geographic region was divided into south and north in China via the address of household registration based on the Yangtze River. Body mass index (BMI) was calculated using weight and height based on the following formula: BMI = weight/height2 (kg/m2), and then categorized the individuals into four groups as follows: underweight (<18.5 kg/m2), normal weight (18.5–23.9 kg/m2), and overweight & obesity (≥24 kg/m2). Female alcohol consumption and husband smoking were self-reported. G6PD deficiency was defined as G6PD enzyme activity below the lower limit of the medical reference range, while elevated creatinine was defined as serum creatinine levels above the upper limit of the medical reference range, separately (Supplementary Table 1). Serum glucose was categorized into the <6.1 mmol/L and ≥6.1 mmol/L groups.
Blood pressure was the main outcome in our study. According to the latest AHA guidelines in 2017 [5], we defined normal blood pressure as SBP < 120 mm Hg and DBP < 80 mm Hg, elevated blood pressure as SBP 120–129 mm Hg and DBP < 80 mm Hg, and hypertension as SBP ≥ 130 mm Hg or DBP ≥ 80 mm Hg.
Statistical analysis
The variables included in this study on the characteristics of the participants in the three groups (normal blood pressure, elevated blood pressure and hypertension) are presented as counts (percentages). χ2 tests were used to examine the differences in categorical variables between the three groups. We used logistic regression to estimate the odds ratios (ORs) and their corresponding 95% confidence intervals (CIs) for the association of G6PD deficiency with elevated blood pressure and hypertension. Multivariable-adjusted logistic regression was employed to adjust for demographic characteristics, lifestyle information, BMI, serum creatinine and glucose.
We further divided SBP and DBP into three groups (SBP groups: < 120, 120–139, and ≥ 140 mm Hg; DBP groups: < 80, 80–89, and ≥ 90 mm Hg) to analyze whether SBP or DBP showed an independent association with G6PD deficiency via multivariable-adjusted logistic regression.
Subgroup analyses were conducted according to demographic characteristics, lifestyle information, BMI, serum creatinine and glucose. Statistical analyses were conducted using Microsoft R Open, version 3.3.3 (Microsoft, Inc).
Results
Characteristics of the study population according to blood pressure status
By 31 December 2016, 154 917 eligible females aged 20–49 had participated in the NFPCP in Shenzhen, China. The prevalence of G6PD deficiency among the participants was 2.09%. The characteristics of the study population are presented according to blood pressure status (Table 1). Females with elevated blood pressure and hypertension were more likely to have the following characteristics: older, non-Han nationality, rural inhabitants, northern residents, greater BMIs, no alcohol consumption, elevated serum creatinine levels, elevated serum glucose levels, and lower educational levels, compared to females with normal blood pressure. Wives with elevated blood pressure and hypertension were more likely to have husband smoking exposure than normal blood pressure wives. In particular, the prevalence of G6PD deficiency was significantly higher in the elevated blood pressure and hypertension groups.
Association between G6PD deficiency and abnormal blood pressure
In our study population, the prevalence of elevated blood pressure and hypertension were 8.68% and 20.14%, respectively. Table 2 presents the ORs of G6PD deficiency with abnormal blood pressure (including elevated blood pressure and hypertension). Participants with G6PD deficiency were positively associated with abnormal blood pressure in both crude and age + BMI-adjusted models. In the multivariable-adjusted model, participants with a G6PD deficiency had an 18% higher risk of elevated blood pressure and an 11% higher risk of hypertension than those without G6PD deficiency. Using different covariates to adjust the model did not substantially influence the OR values, which were similar in the three models. It was reported that G6PD deficiency was associated with diabetes [31]; thus, we used serum glucose as a covariate factor to adjust the model, and the results were still statistically significant (adjusted ORs were 1.14 (95% CI: 1.01–1.28) for elevated blood pressure and 1.11 (95% CI: 1.02–1.21) for hypertension respectively).
Association of G6PD deficiency with systolic blood pressure and diastolic blood pressure
We further explored the association of G6PD deficiency with SBP and DBP. The crude ORs, age and BMI-adjusted ORs, and multivariable-adjusted ORs are presented in Table 3. Compared with participants without G6PD deficiency, the crude ORs for the SBP 120–139 mm Hg and SBP ≥ 140 mm Hg groups were 1.12 (95% CI: 1.03–1.21) and 1.36 (95% CI: 1.05–1.76), respectively. After adjusting for age and BMI, the ORs were 1.12 (95% CI: 1.03–1.21) and 1.38 (95% CI: 1.06–1.80), respectively. The multivariable-adjusted ORs for G6PD deficiency were 1.10 (95% CI: 1.00–1.21) and 1.74 (95% CI: 1.25–2.42), respectively. All three models showed a positive association between G6PD deficiency and elevated SBP. For the group with DBP ranging from 80–89 mm Hg, the crude OR, age and BMI-adjusted OR, and multivariable-adjusted OR were 1.09 (95% CI: 1.00–1.20), 1.10 (95% CI: 1.00–1.20), and 1.09 (95% CI: 0.98–1.21), respectively, compared with females without G6PD deficiency in the DBP < 80 mm Hg group. However, when DBP ≥ 90 mm Hg, the ORs of all three models showed no statistical significance.
Subgroup analysis of the association between G6PD deficiency and abnormal blood pressure
The results from the subgroup analyses are shown in Fig. 2. We performed logistic regression analysis on age, nationality, urban/rural inhabitants, southern/northern residents, educational level, alcohol consumption, BMI, serum creatinine, serum glucose, and husband smoking separately. The results showed that the multivariable-adjusted ORs in most subgroups were consistent.
Discussion
G6PD deficiency is the most common metabolic disorder of red blood cells [32] as well as the most widespread form of acute hemolytic anemia [33]. Both male hemizygotes and female homozygotes with the G6PD gene deficiency manifest as significantly lower G6PD enzyme activity, while 80% of female heterozygotes manifest as intermediate lower G6PD enzyme activity [34]. In general, G6PD deficiency includes all male hemizygotes and female homozygotes with a G6PD gene deficiency, as well as a small amount of female heterozygotes whose G6PD enzyme activity manifests as significantly lower levels; however, it does not include the vast majority of female heterozygotes whose G6PD enzyme activity manifests as intermediately low or normal [34, 35]. This results in a higher prevalence of G6PD deficiency in males than in females. The WHO Working Group recommends that the diagnosis of G6PD deficiency in adults by quantitative estimation of the enzyme activity levels in red cells is easy and desirable at the public health level [36]. The molecular diagnostic test is not recommended for use in a large population, as the primers employed are not comprehensive enough to identify all cases of G6PD deficiency, and it requires sophisticated equipment and highly trained human resources [37, 38]. Therefore, we defined G6PD deficiency by quantitative estimation of the G6PD enzyme activity levels in this study.
In the past, most studies on G6PD deficiency focused on the male population. Now, more and more researchers believe that the female population, especially female heterozygotes, should not be ignored [11]. According to the Developmental Origins of Health and Disease theory, in addition to adulthood lifestyle and genetic factors, early life (prepregnancy, pregnancy, infancy and childhood), the interplay between maternal and environmental factors (induce physiological changes), and fetal and child growth and development have long-term consequences on later health and noncommunicable disease risk (such as cardiovascular disease, obesity, type II diabetes and metabolic disturbances). Timely interventions may reduce this risk in individuals and limit its transmission to the next generation [39]. Existing literature has reported that hypertensive disorders in pregnancy had an overall negative impact on the offspring’s cardiovascular, immune and neurological health [40], while G6PD deficiency and carriers of G6PD deficiency were more likely to have spontaneous abortion, low birth weight [41], intrauterine fetal death, stillbirth, fetal abnormalities and other adverse pregnancy outcomes [42]. Therefore, we tried to explore the relationship between G6PD deficiency and abnormal blood pressure among prepregnant reproductive-age females to provide references for reducing the incidence of adverse pregnancy outcomes and improving maternal and child health.
This large population-based study showed that females aged 20–49 with G6PD deficiency had a higher risk of elevated blood pressure and hypertension. Based on the data stratification for systolic blood pressure and diastolic blood pressure, we found that G6PD deficiency was more obviously associated with systolic blood pressure. The results of the subgroup analysis showed the same conclusion.
Few studies have reported the association between G6PD deficiency and elevated blood pressure or hypertension, and findings from some studies were consistent with ours. In Stephen L’s study [22], comparison of 55 G6PD-deficient black men’s physical and laboratory data with 1154 G6PD-normal participants using quantitative data in the clinic showed that black people with G6PD deficiency were more likely to have slightly elevated blood pressure. We further extended the study with a large population data and analyzed the association of G6PD deficiency with elevated blood pressure and hypertension, as well as its association with systolic blood pressure and diastolic blood pressure. Most researchers explain the hypertension mechanism as a result of an antioxidant deficiency [43], and G6PD deficiency is likely to lower antioxidant levels [44], which could be a possible pathway to elevate blood pressure and hypertension [19, 44]. Filosa S[45] suggested that G6PD was the only NADPH-producing enzyme activated in response to oxidative stress that could act as a guardian of the cell redox potential and protect organs from free radical damage. Robert St. C. Gaskin’s study [46] suggested that G6PD deficiency was a cause of both oxidative stress and a decrease in the generation of nitric oxide (NO). The former is considered as a mechanism leading to hypertension, while the latter might give rise to vasospasm, which is a possible way to raise blood pressure. These findings support that G6PD-deficient individuals are vulnerable to oxidative stress and more likely to show elevated blood pressure and hypertension. Moreover, Al-Abdi suggested that G6PD deficiency might be associated with reduced glutathione S-transferase (GST) [47], while Polimanti R suggested that reduced GST levels might be associated with essential hypertension [48]. In other words, reducing GST may be another plausible biological mechanism that could explain the founded association of G6PD deficiency with elevated blood pressure and hypertension.
However, the findings from some studies were not exactly the same as ours. For example, a study conducted in Nigeria found that compared with 207 nondeficient males, 52 G6PD deficiency males’ systolic blood pressure was slightly elevated while diastolic blood pressure was slightly reduced. Meanwhile, compared with 65 noncarrier females, 5 carrier females’ systolic and diastolic blood pressure were slightly reduced, though none of the results showed statistical significance [23]. The main reason might be that the two studies differ in their demographics (gender, race, average age, etc.), sample size and statistical methods. There were also some inconsistencies in the animal mechanism research. Matsui et al [18] compared blood pressure in hemizygote G6PD mutant and wild-type (WT) C3H mice after Ang II infusion and found that G6PD deficiency might provide an advantage by protecting against Ang II-dependent hypertension by decreasing the contribution of NADPH oxidase-derived superoxide anion to the pressor and hypertrophic vascular response, which meant that G6PD deficiency might protect vessel reconstruction and have the potential to prevent hypertension. However, whether the discrepancy was caused by species differences or by chance requires additional research in the future.
On the basis of the data stratification of systolic and diastolic blood pressure, we found that the association between G6PD deficiency and systolic blood pressure was more obvious. Díaz-Flores M’s study [49] suggested that oral supplementation with glycine (an antioxidant) could increase G6PD (an oxidative stress marker) activity and lower systolic blood pressure, which meant that increasing antioxidant levels in the blood could partly improve G6PD deficiency and decrease systolic blood pressure. In terms of mechanistic studies, Jane A. Leopold [50] found that vascular smooth muscle cells might be subjected to mechanical forces, such as cyclic strain, that promote the formation of reactive oxygen species, but that cells could modulate this adverse milieu by increasing the expression of G6PD to maintain or restore intracellular glutathione levels. This means that G6PD could protect vascular smooth muscle cells from damage and prevent vessel stiffness. Michael J. Domanski [51] suggested that increased conduit vessel stiffness could result in increased characteristic impedance of the aorta and decreased arterial compliance, which could cause an increase in systolic blood pressure as well as a decrease in diastolic blood pressure. These results support that G6PD deficiency might increase systolic blood pressure by causing vessel stiffness through oxidative stress. However, the association between G6PD deficiency and diastolic blood pressure showed no statistical significance in our study; thus, more in-depth studies are needed in the future.
We believe our research has important public health and clinical implications because both G6PD deficiency and hypertension (or elevated blood pressure) populations are very large. The definition of hypertension used in this study was based on new guidelines by the AHA in 2017. The new guideline lowered the cutoff value of hypertension and removed the concept of prehypertension (which was divided into elevated blood pressure and stage I hypertension), which was the main difference from the JNC7 criteria [4, 5]. This reflected a major and important shift in the management of hypertension. More people would be labeled as having hypertension, though not all of them should be prescribed antihypertensive medicines because the risk of cardiovascular disease also needs to be considered. These guidelines mainly affect young people’s blood pressure management and emphasize the importance of early interventions for hypertension. Our study was conducted in a young population (average age was 28.77 and 64.15% of females were aged 20–29) and illustrated that females with G6PD deficiency had a higher risk of elevated blood pressure and hypertension. It was reported that females with elevated blood pressure could be more likely to get hypertensive disorders during pregnancy [6], which could result in adverse pregnancy outcomes (such as preterm labor, birth defects, and neonatal death) [7, 52] and maternal death [8]. Since G6PD deficiency is a risk factor for preeclampsia (a hypertensive disorder in pregnancy) [53] and G6PD deficiency is an X-linked genetic disease [9], reproductive-age females with G6PD deficiency combined with elevated blood pressure or hypertension are a high-risk population, especially during the prepregnancy and pregnancy periods. Hence, early interventions such as a low-salt diet [54] and exercise should be strengthened for prepregnant females with G6PD deficiency combined with abnormal blood pressure to reduce the risk of hypertensive disorders during pregnancy [53], as well as consequent vascular complications and metabolic syndrome [49]. In contrast, strengthening G6PD screening programs in newborns and prepregnant couples in areas where the prevalence of G6PD deficiency and hypertension are both high could reduce the burden of these two diseases and adverse pregnancy outcomes.
Based on our knowledge, this was the first large-scale population study to explore the association of G6PD deficiency with elevated blood pressure and hypertension in prepregnancy females in China. This study was based on standard data collection methods and strict laboratory quality control to ensure the reliability of the data. The results illustrated that females with G6PD deficiency had a higher risk of elevated blood pressure and hypertension (particularly systolic blood pressure). However, some limitations should also be declared. First, there were two kinds of quantitative estimation of G6PD enzyme activity screening methods at medical organizations included in this study; thus, we defined G6PD deficiency as a categorical variable according to whether G6PD enzyme activity was below the lower limit of the medical reference range. Thus, we could not stratify G6PD enzyme activity into more specific groups for quantitative analysis or show the dose-response relationship between G6PD enzyme activity and blood pressure. However, the results from the quantitative estimation of G6PD enzyme activity with the two screening methods were similar [55], and the laboratory quality control in this study was very strict, which made us believe it was reasonable. Second, we only used a single test of blood pressure in the resting state to define elevated blood pressure and hypertension, and information on antihypertensive drug use was not collected when defining hypertension status, which might also cause misclassification bias. However, those without hypertension were less likely to take antihypertensive medication, which minimized the likelihood of misclassification bias. Third, participants in this study were females who underwent prepregnancy physical examinations and might be healthier than the general population. This difference might introduce an underestimation of the risk in the general population. Lastly, we performed a cross-sectional study to prove that the causal relationship between G6PD deficiency and elevated blood pressure was unconvincing. Therefore, prospective studies should be conducted to further verify the causal relationship, and more mechanisms should be explored in the future to explain the associations between the two diseases.
References
Wang J, Zhang L, Wang F, Liu L, Wang Haiyan. Prevalence, awareness, treatment, and control of hypertension in China: results From a National Survey. Am J Hypertens. 2014;27:1355–61.
Liang Y, Liu R, Du S, Qiu C. Trends in incidence of hypertension in Chinese adults, 1991–2009: The China Health and Nutrition Survey. Int J Cardiol. 2014;175:196–01.
Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension. 2008;52:818–27.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. National Heart, lung, and blood institute joint national committee on prevention, detection, evaluation, and treatment of high blood pressurenational high blood pressure education program coordinating committee. the seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72.
Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017;71:1269–324.
Black MH, Zhou H, Sacks DA, Dublin S, Lawrence JM, Harrison TN, et al. Prehypertension prior to or during early pregnancy is associated with increased risk for hypertensive disorders in pregnancy and gestational diabetes. J Hypertens. 2015;33:1860–1867.
Yi-qun W. Risk factor related to hypertensive disorders in pregnancy and effects of hypertension on pergnancy outcome. Chin J General Pract. 2015;13:602–4.
Ding D, Dan Z, Shao-ping Y, Meng-jie C, Duo-sheng J, Bi-fang P. Analysis on maternal death and intervention measures from 2005 to 2007 in Wuhan. Chin J Matern Child Health. 2010;25:4176–8.
Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371:64–74.
Gotsman I, Muszkat M. Glucose-6-phosphate dehydrogenase deficiency is associated with increased initial clinical severity of acute viral hepatitis A. J Gastroenterol Hepatol. 2001;16:1239–43.
Albayrak C, Albayrak D. Red cell glucose-6-phosphate dehydrogenase deficiency in the northern region of Turkey: Is G6PD deficiency exclusively a male disease? Pediatr Hematol Oncol. 2015;32:85–91.
Yang H, Wang Q, Zheng L, Zhan XF, Lin M, Lin F, et al. Incidence and molecular characterization of Glucose-6-Phosphate Dehydrogenase deficiency among neonates for newborn screening in Chaozhou, China. Int J Lab Hematol. 2015;37:410–9.
National health and Family Planning commision. Department of maternal and child health service in Chinese National health and Family Planning commision. The annual report of maternal and child health monitoring in 2017: National health and Family Planning commision, 2017. https://www.mchscn.org/admin/xiazai/tongxun/2017%E5%B9%B4%E5%85%A8%E5%9B%BD%E5%A6%87%E5%B9%BC%E5%8D%AB%E7%94%9F%E7%9B%91%E6%B5%8B%E5%8F%8A%E5%B9%B4%E6%8A%A5%E9%80%9A%E8%AE%AF%E7%AC%AC2%E6%9C%9F.pdf.
National health and Family Planning commision. Department of maternal and child health service in Chinese National health and Family Planning commision. The annual report of maternal and child health monitoring in 2016: National health and Family Planning commision, 2016. https://www.mchscn.org/admin/xiazai/tongxun/2016%E5%B9%B4%E5%85%A8%E5%9B%BD%E5%A6%87%E5%B9%BC%E5%8D%AB%E7%94%9F%E7%9B%91%E6%B5%8B%E5%8F%8A%E5%B9%B4%E6%8A%A5%E9%80%9A%E8%AE%AF%E7%AC%AC3%E6%9C%9F.pdf.
Vizzi E, Bastidas G, Hidalgo M, Colman L, Pérez HA. Prevalence and molecular characterization of G6PD deficiency in two Plasmodium vivax endemic areas in Venezuela: predominance of the African A-202A/376G variant. Malar J. 2016;15:19.
Mahmoud AA, Nor ElDin AK. Glucose-6-phosphate dehydrogenase activity and protein oxidative modification in patients with type 2 diabetes mellitus. J Biomark. 2013;2013:430813.
Zhang Z, Liew CW, Handy DE, Zhang Y, Leopold JA, Hu J, et al. High glucose inhibits glucose-6-phosphate dehydrogenase, leading to increased oxidative stress and beta-cell apoptosis. FASEB J. 2010;24:1497–505.
Matsui R, Xu S, Maitland KA, Hayes A, Leopold JA, Handy DE, et al. Glucose-6 phosphate dehydrogenase deficiency decreases the vascular response to angiotensin II. Circulation. 2005;112:257–63.
Touyz RM, Briones AM. Reactive oxygen species and vascular biology: implications in human hypertension. Hypertens Res. 2011;34:5–14.
Solak Y, Afsar B, Vaziri ND, Aslan G, Yalcin CE, Covic A, et al. Hypertension as an autoimmune and inflammatory disease. Hypertens Res. 2016;39:567–73.
Niazi ZR, Silva GC, Ribeiro TP, Leon-Gonzalez AJ, Kassem M, Mirajkar A, et al. EPA:DHA 6:1 prevents angiotensin II-induced hypertension and endothelial dysfunction in rats: role of NADPH oxidase- and COX-derived oxidative stress. Hypertens Res. 2017;40:966–75.
Wiesenfeld SL, Petrakis NL, Sams BJ, Collen MF, Cutler JL. Elevated blood pressure, pulse rate and serum creatinine in Negro males deficient in glucose-6-phosphate dehydrogenase. N Engl J Med. 1970;282:1001–2.
Nwankwo MU, Bunker CH, Ukoli FA, Omene JA, Freeman DT, Vergis EN, et al. Blood pressure and other cardiovascular disease risk factors in black adults with sickle cell trait or glucose-6-phosphate dehydrogenase deficiency. Genet Epidemiol. 1990;7:211–8.
Shenzhen Bureau of Statistics. Satastical report of social sex characters of Shenzhen in 2015. http://www.sztj.gov.cn/xxgk/tjsj/tjgb/201707/t20170714_7876524.htm. Accessed 4 Sep 2017.
Shenzhen Statistical Bureau. The Sixth nationwide population census report of Shenzhen in 2010. http://www.sztj.gov.cn/xxgk/tjsj/pcgb/201105/t20110512_2061597.htm. Accessed 4 Sep 2017.
Zhongling C, xinyan W, Meizhen F, JIanhua L, Shuangqing G, Shu L, et al. Glucose-6-phosphate deficiency screening of reproductive population in Baoan District. Chin J Reprod Heal. 2006;17:240.
Zhi-li W, Xiao-li H, Wei-yong Z. Analysis on screening of G6PD deficiency among 3465 fetile women in Longgang district of Shenzhen city. Ji Lin Yi Xue. 2011;32:2616–7.
Zhang S, Wang Q, Shen H. The design, implementation and significance of National Free Pre-conception Check-up Projects in China. Natl Med J China. 2015;95:162–5.
Yang Y, Liu F, Wang L, Li Q, Wang X, Chen JC, et al. Association of husband smoking with wife’s hypertension status in over 5 million chinese females aged 20 to 49 years. J Am Heart Assoc. 2017;6:e004924.
Zhou Q, Zhang S, Wang Q, Shen H, Tian W, Chen J, et al. China’s community-based strategy of universal preconception care in rural areas at a population level using a novel risk classification system for stratifying couples´ preconception health status. BMC Health Serv Res. 2016;16:689.
Lai YK, Lai NM, Lee SW. Glucose-6-phosphate dehydrogenase deficiency and risk of diabetes: a systematic review and meta-analysis. Ann Hematol. 2017;96:839–45.
Nabavizadeh SH, Anushiravani A. The prevalence of G6PD deficiency in blood transfusion recipients. Hematol (Amst, Neth). 2007;12:85–8.
Luzzatto L, Arese P. Favism and glucose-6-phosphate dehydrogenase deficiency. New Engl J Med. 2018;378:60–71.
Kaplan M, Hammerman C. Neonatal screening for glucose-6-phosphate dehydrogenase deficiency: biochemical versus genetic technologies. Semin Perinatol. 2011;35:155–61.
Johnson MK, Clark TD, Njama-Meya D, Rosenthal PJ, Parikh S. Impact of the method of g6pd deficiency assessment on genetic association studies of malaria susceptibility. PLoS ONE. 2009;4:e7246.
World Health Organization. Glucose-6-phosphate dehydrogenase deficiency. WHO Working Group. Bull World Health Organ. 1989;67:601–11.
Howes RE, Battle KE, Satyagraha AW, Baird JK, Hay SI. G6PD deficiency: global distribution, genetic variants and primaquine therapy. Adv Parasitol. 2013;81:133–201.
Watchko JF, Kaplan M, Stark AR, Stevenson DK, Bhutani VK. Should we screen newborns for glucose-6-phosphate dehydrogenase deficiency in the United States? J Perinatol. 2013;33:499–504.
Gluckman PD, Hanson MA. Developmental origins of disease paradigm: a mechanistic and evolutionary perspective. Pediatr Res. 2004;56:311.
Pinheiro TV, Brunetto S, Ramos JG, Bernardi JR, Goldani MZ. Hypertensive disorders during pregnancy and health outcomes in the offspring: a systematic review. J Dev Orig Health Dis. 2016;7:391–407.
Toncheva D, Tzoneva M. Prenatal selection and fetal development disturbances occurring in carriers of G6PD deficiency. Hum Genet. 1985;69:88.
Nicol CJ, Zielenski J, Tsui LC, Wells PG. An embryoprotective role for glucose-6-phosphate dehydrogenase in developmental oxidative stress and chemical teratogenesis. Faseb J. 2000;14:111–27.
Redón J, Oliva MR, Tormos C, Giner V, Chaves J, Iradi A, et al. Antioxidant activities and oxidative stress byproducts in human hypertension. Hypertension. 2003;41:1096–101.
Nóbregapereira S, Fernandezmarcos PJ, Brioche T, Gomezcabrera MC, Salvadorpascual A, Flores JM, et al. G6PD protects from oxidative damage and improves healthspan in mice. Nat Commun. 2016;7:10894.
Filosa S, Annalisa F, Paglialunga F, Balestrieri M, Crooke A, Verde P, et al. Failure to increase glucose consumption through the pentose-phosphate pathway results in the death of glucose-6-phosphate dehydrogenase gene-deleted mouse embryonic stem cells subjected to oxidative stress. Biochem J. 2003;370:935–43.
Gaskin RS, Estwick D, Peddi R. G6PD deficiency: its role in the high prevalence of hypertension and diabetes mellitus. Ethn & Dis. 2001;11:749–54.
Al-Abdi SY. Decreased glutathione s-transferase level and neonatal hyperbilirubinemia associated with glucose-6-phosphate dehydrogenase deficiency: a perspective review. Am J Perinatol. 2017;34:305–14.
Polimanti R, Piacentini S, Lazzarin N, Re MA, Manfellotto D, Fuciarelli M. Glutathione S-transferase variants as risk factor for essential hypertension in Italian patients. Mol Cell Biochem. 2011;357:227–33.
Díaz-Flores M, Cruz M, Duran-Reyes G, Munguia-Miranda C, Loza-Rodríguez H, Pulido-Casas E, et al. Oral supplementation with glycine reduces oxidative stress in patients with metabolic syndrome, improving their systolic blood pressure. Can J Physiol & Pharmacol. 2013;91:855–60.
Leopold JA, Loscalzo J. Cyclic strain modulates resistance to oxidant stress by increasing G6PDH expression in smooth muscle cells. Am J Physiol Heart Circ Physiol. 2000;279:H2477–85.
Domanski MJ, Davis BR, Pfeffer MA, Kastantin M, Mitchell GF. Isolated systolic hypertension. Progn Inf Provid Pulse Press. 1999;34:375–80.
Feng YL, Peng TT, Wang F, Tong SU, Yue FJ, Zhang LR, Zhang YW, Yang HL, Wang SP. Impact of hypertensive disorder complicating pregnancy on birth outcomes and its potential risk factors. Chinese Journal of Disease Control & Prevention 2014;18:131–34.
Abdulhadi NH. Glucose 6 phosphate dehydrogenase (G6PD) deficiency is a possible risk factor for the development of preeclampsia. Med Hypotheses. 2004;62:780–2.
Liu Z, Qi H, Liu B, Liu K, Wu J, Cao H, et al. Genetic susceptibility to salt-sensitive hypertension in a Han Chinese population: a validation study of candidate genes. Hypertens Res. 2017;40:876–84.
Chunlin X. Comparation of two G6PD enzyme test system. Chin Prac Med. 2010;05:63–64.
Acknowledgements
This study was supported by the 13th Five-Year Plan, National Key Research and Development Program of China (grants No. 2016YFC1000300 and 2016YFC1000307), the National Human Genetic Resources Sharing Service Platform (grant No.2005DKA21300) and the National Population and Reproductive Health Science Data Center (grant No.2005DKA32408), People’s Republic of China. We would like to express our sincere gratitude to the health workers and participants throughout Shenzhen in the NFPCP for their great efforts and collaboration.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhao, J., Zhang, X., Guan, T. et al. The association between glucose-6-phosphate dehydrogenase deficiency and abnormal blood pressure among prepregnant reproductive-age Chinese females. Hypertens Res 42, 75–84 (2019). https://doi.org/10.1038/s41440-018-0118-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-018-0118-1