Abstract
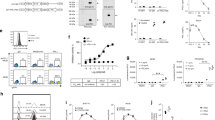
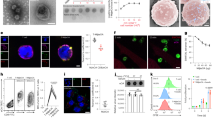
Oncolytic reovirus administration has been well tolerated by cancer patients in clinical trials. However, its anti-cancer efficacy as a monotherapy remains to be augmented. We and others have previously demonstrated the feasibility of producing replication-competent reoviruses expressing a heterologous transgene. Here, we describe the production of recombinant reoviruses expressing murine (mm) or human (hs) GM-CSF (rS1-mmGMCSF and rS1-hsGMCSF, respectively). The viruses could be propagated up to 10 passages while deletion mutants occurred only occasionally. In infected cell cultures, the secretion of GM-CSF protein (up to 481 ng/106 cells per day) was demonstrated by ELISA. The secreted mmGM-CSF protein was functional in cell culture, as demonstrated by the capacity to stimulate the survival and proliferation of the GM-CSF-dependent dendritic cell (DC) line D1, and by its ability to generate DCs from murine bone marrow cells. Importantly, in a murine model of pancreatic cancer we found a systemic increase in DC and T-cell activation upon intratumoral administration of rS1-mmGMCSF. These data demonstrate that reoviruses expressing functional GM-CSF can be generated and have the potential to enhance anti-tumor immune responses. The GM-CSF reoviruses represent a promising new agent for use in oncolytic virotherapy strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Keller BA, Bell JC. Oncolytic viruses-immunotherapeutics on the rise. J Mol Med. 2016;94:979–91.
Hu JC, Coffin RS, Davis CJ, Graham NJ, Groves N, Guest PJ, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 2006;12:6737–47.
Pol J, Kroemer G, Galluzzi L. First oncolytic virus approved for melanoma immunotherapy. Oncoimmunology. 2016;5:e1115641.
Zhao X, Chester C, Rajasekaran N, He Z, Kohrt HE. Strategic combinations: the future of oncolytic virotherapy with reovirus. Mol Cancer Ther. 2016;15:767–73.
Komoto S, Kawagishi T, Kobayashi T, Ikizler M, Iskarpatyoti J, Dermody TS, et al. A plasmid-based reverse genetics system for mammalian orthoreoviruses driven by a plasmid-encoded T7 RNA polymerase. J Virol Methods. 2014;196:36–9.
van den Wollenberg DJ, Dautzenberg IJ, Ros W, Lipinska AD, van den Hengel SK, Hoeben RC. Replicating reoviruses with a transgene replacing the codons for the head domain of the viral spike. Gene Ther. 2015;22:267–79.
van den Wollenberg DJ, Dautzenberg IJ, van den Hengel SK, Cramer SJ, de Groot RJ, Hoeben RC. Isolation of reovirus T3D mutants capable of infecting human tumor cells independent of junction adhesion molecule-A. PLoS ONE 2012;7:e48064.
Kemp V, Dautzenberg IJC, Cramer SJ, Hoeben RC, van den Wollenberg DJM. Characterization of a replicating expanded tropism oncolytic reovirus carrying the adenovirus E4orf4 gene. Gene Ther. 2018;25:331–34.
Branton PE, Roopchand DE. The role of adenovirus E4orf4 protein in viral replication and cell killing. Oncogene. 2001;20:7855–65.
Marcellus RC, Chan H, Paquette D, Thirlwell S, Boivin D, Branton PE. Induction of p53-independent apoptosis by the adenovirus E4orf4 protein requires binding to the Balpha subunit of PP2A. J Virol. 2000;74:7869–77.
Xu Y, Zhan Y, Lew AM, Naik SH, Kershaw MH. Differential development of murine dendritic cells by GM-CSF versus Flt3 ligand has implications for inflammation and trafficking. J Immunol. 2007;179:7577–84.
Zhan Y, Carrington EM, van Nieuwenhuijze A, Bedoui S, Seah S, Xu Y, et al. GM-CSF increases cross-presentation and CD103 expression by mouse CD8(+) spleen dendritic cells. Eur J Immunol. 2011;41:2585–95.
Fallaux FJ, Kranenburg O, Cramer SJ, Houweling A, Van Ormondt H, Hoeben RC, et al. Characterization of 911: a new helper cell line for the titration and propagation of early region 1-deleted adenoviral vectors. Hum Gene Ther. 1996;7:215–22.
van den Wollenberg DJ, van den Hengel SK, Dautzenberg IJ, Cramer SJ, Kranenburg O, Hoeben RC. A strategy for genetic modification of the spike-encoding segment of human reovirus T3D for reovirus targeting. Gene Ther. 2008;15:1567–78.
Lee JW, Komar CA, Bengsch F, Graham K, Beatty GL. Genetically engineered mouse models of pancreatic cancer: the KPC model (LSL−Kras(G12D/+);LSL−Trp53(R172H/+);Pdx-1-Cre), its variants, and their application in immuno-oncology drug discovery. Curr Protoc Pharmacol. 2016;73:14.39.1–14.39.20.
Buchholz UJ, Finke S, Conzelmann KK. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J Virol. 1999;73:251–9.
Winzler C, Rovere P, Rescigno M, Granucci F, Penna G, Adorini L, et al. Maturation stages of mouse dendritic cells in growth factor–dependent long-term cultures. J Exp Med. 1997;185:317–28.
Boross P, van Montfoort N, Stapels DA, van der Poel CE, Bertens C, Meeldijk J, et al. FcRgamma-chain ITAM signaling is critically required for cross-presentation of soluble antibody-antigen complexes by dendritic cells. J Immunol. 2014;193:5506–14.
Roner MR, Roehr J. The 3’ sequences required for incorporation of an engineered ssRNA into the Reovirus genome. Virol J. 2006;3:1.
Mijatovic-Rustempasic S, Tam KI, Kerin TK, Lewis JM, Gautam R, Quaye O, et al. Sensitive and specific quantitative detection of rotavirus A by one-step real-time reverse transcription-PCR assay without antecedent double-stranded-RNA denaturation. J Clin Microbiol. 2013;51:3047–54.
Helft J, Bottcher J, Chakravarty P, Zelenay S, Huotari J, Schraml BU, et al. GM-CSF mouse bone marrow cultures comprise a heterogeneous population of CD11c(+)MHCII(+) macrophages and dendritic cells. Immunity. 2015;42:1197–211.
Eaton HE, Kobayashi T, Dermody TS, Johnston RN, Jais PH, Shmulevitz M. African swine fever virus NP868R capping enzyme promotes reovirus rescue during reverse genetics by promoting reovirus protein expression, virion assembly, and RNA incorporation into infectious virions. J Virol. 2017;91:e02416–16.
Author information
Authors and Affiliations
Contributions
VK, DJMvdW, MGMC, TvH, PK, NPvM, and RCH all compiled data and jointly wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kemp, V., van den Wollenberg, D.J.M., Camps, M.G.M. et al. Arming oncolytic reovirus with GM-CSF gene to enhance immunity. Cancer Gene Ther 26, 268–281 (2019). https://doi.org/10.1038/s41417-018-0063-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-018-0063-9
This article is cited by
-
Engineering strategies to enhance oncolytic viruses in cancer immunotherapy
Signal Transduction and Targeted Therapy (2022)
-
Gastrointestinal cancer-associated fibroblasts expressing Junctional Adhesion Molecule-A are amenable to infection by oncolytic reovirus
Cancer Gene Therapy (2022)
-
Oncolytic Viral Therapy for Malignant Glioma and Their Application in Clinical Practice
Neurotherapeutics (2022)
-
Immunotherapy for pancreatic cancer: chasing the light at the end of the tunnel
Cellular Oncology (2021)