Abstract
Perinatal brain injury is the leading cause of neurological mortality and morbidity in childhood ranging from motor and cognitive impairment to behavioural and neuropsychiatric disorders. Various noxious stimuli, including perinatal inflammation, chronic and acute hypoxia, hyperoxia, stress and drug exposure contribute to the pathogenesis. Among a variety of pathological phenomena, the unique developing immune system plays an important role in the understanding of mechanisms of injury to the immature brain. Neuroinflammation following a perinatal insult largely contributes to evolution of damage to resident brain cells, but may also be beneficial for repair activities. The present review will focus on the role of peripheral immune cells and discuss processes involved in neuroinflammation under two frequent perinatal conditions, systemic infection/inflammation associated with encephalopathy of prematurity (EoP) and hypoxia/ischaemia in the context of neonatal encephalopathy (NE) and stroke at term. Different immune cell subsets in perinatal brain injury including their infiltration routes will be reviewed and critical aspects such as sex differences and maturational stage will be discussed. Interactions with existing regenerative therapies such as stem cells and also potentials to develop novel immunomodulatory targets are considered.
Impact
-
Comprehensive summary of current knowledge on the role of different immune cell subsets in perinatal brain injury including discussion of critical aspects to be considered for development of immunomodulatory therapies.
Similar content being viewed by others
Introduction
Perinatal insults resulting in brain injury may affect preterm and also term-born babies. Various noxious stimuli, including perinatal infection yielding excess inflammation, foetal hypoxia, intrapartum oxygen deprivation, hyperoxia, stress from maternal separation and exposure to medication or anaesthesia, increase the risk for long-lasting neurological impairment. In the present review, we will focus on two frequent perinatal conditions, systemic infection/inflammation associated with encephalopathy of prematurity (EoP) and hypoxia/ischaemia in the context of neonatal encephalopathy (NE) and stroke at term. A large body of clinical research investigated biomarkers of injury in order to determine infants at risk for injury and adverse brain maturation. Experimental research concentrated on underlying mechanisms in order to identify targets for neuroprotection and/or induction of repair with the overall aim of personalized therapy for each child.
NE following asphyxia in term infants affects approximately 1.5–3 in 1000 live births in high-income countries1,2 and approximately 10–20 of 1000 in low- and middle-income countries2,3. Though introduction of hypothermia treatment (HT) into standard clinical practise significantly improved neurodevelopmental outcome, mortality remains high and a significant proportion of infants with NE still suffers from long-lasting motor-cognitive impairment. Children with neonatal acute ischaemic stroke, with an incidence of 7.1–16 in 100,000 live births, are also at high risk for poor neurological outcome4,5,6,7. The most commonly used experimental model for neonatal asphyxia has been established in rodents by Rice and Vannucci in 1981 with unilateral common carotid artery occlusion followed by systemic hypoxia (hypoxia–ischaemia, HI)8. The most commonly used neonatal stoke model is the occlusion of the middle cerebral artery9,10. While initially developed in postnatal day (PND) 7 rodents8,9, corresponding to brain development of late preterms, models have been adapted to 9–10-day-old animals to mimic hypoxic and ischaemic insults at term11,12,13. Pathophysiological processes involved in hypoxic/ischaemic brain injury include excitotoxicity, apoptosis, inflammation and subacute white and grey matter injury, resulting in long-lasting brain atrophy mainly affecting cortex, hippocampus and striatum8,10,13,14,15.
In preterm infants suffering EoP, early- and late-onset infections with specific patterns of inflammatory markers have been linked to adverse outcome, including development of motor and/or cognitive impairment, with cerebral palsy as the worst outcome16,17,18. Several experimental models have been developed to mimic inflammation-related development of EoP, e.g., low-dose lipopolysaccharide (LPS) administration on PND3 or repetitive injections of IL-1beta over the first five PNDs. Both injury paradigms lead to a significant impairment of recognition memory, associated with long-lasting alterations of the white matter microstructure19,20. Furthermore, previous clinical studies reported higher infection rates in asphyxiated newborns21 and an increased risk of brain injury in infected infants with NE22. This lead to the development of rodent and large animal models, combining both, systemic inflammation and hypoxic–ischaemic brain injury23,24,25,26,27,28,29,30. While the majority of studies applied LPS, the cell wall component of the Gram-negative bacterium Escherichia coli, which binds to the Toll-like receptor 4 (TLR4), novel combination models mimicking infections with Gram-positive bacteria, have been recently described. In this regard, the synthetic lipoprotein Pam3CSK4 or infection with live Staphylococcus epidermidis, both activating TLR2 signalling are used24,31,32,33. Even though both, TLR4 and TLR2 activation sensitize the brain to increased injury following HI23,28,29,31,32,33, different inflammatory responses are triggered in the brain34. Furthermore, responses to living infectious organisms vs. Toll-like receptor ligands may differ. For instance, Staphylococcus epidermidis bacteremia induces TLR2-dependent but also -independent pathways, which is supported by the fact that systemic bacterial infection impaired brain development in the absence of bacterial entry into the cerebrospinal fluid (CSF)35.
During the perinatal period, both systemic inflammation/infection and hypoxic–ischaemic events induce complex responses in brain-resident immune cells, i.e. microglia, which has been excellently reviewed in several articles36,37,38,39 and was therefore out of the scope of this paper. Here, we particularly focus on the role of peripheral immune cells in the disease paradigms described above. For a long time, the neonatal immune system has been believed to be immature and impaired in function, with an increased risk for sepsis, reduced responses to certain vaccinations and an increased bias towards differentiation into anti-inflammatory Th2 CD4 T cells40,41. However, this concept is challenged by clinical observations of hyper-inflammatory courses of neonatal sepsis42. Recent work suggested that neonatal immune responses are not impaired, but rather determined by a tightly regulated physiological immune programming preventing harmful hyper-inflammation in combination with sufficient immunological protection43. The potency of the neonatal immune system to elicit a strong reaction to dangerous stimuli is also demonstrated by the fact that neonatal peripheral immune cells participate in sterile tissue inflammation induced by perinatal brain injury44, indicating a complex interplay between the peripheral immune and the central nervous system. While the significance of different immune cell subtypes in the development of secondary neurodegeneration has been well established in adult brain injury, e.g. in the context of ischaemic stroke45, this research field is still developing in neonatology.
In the present review, we will (1) summarize current knowledge on the role of different leucocyte subsets in perinatal brain injury, (2) provide an overview on different infiltration routes to the injured brain, (3) emphasize the impact of sex, (4) highlight the important aspect of maturational stage and (5) discuss implications for immunomodulatory therapies.
Divergent role of the various leucocyte subsets in perinatal brain injury
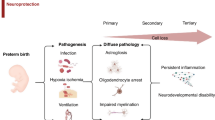
The essential contribution of the peripheral immune system to the development of perinatal brain injury has been initially described in rodent experiments. Splenectomy prior to HI-induced brain injury was associated with pronounced neuroprotection46. These data suggested a detrimental role of peripheral immune cells per se. However, emerging evidence supports that perinatal brain injury, induced by HI and/or stimulation of the peripheral immune system, leads to activation and cerebral infiltration of different immune cell subpopulations, mediating either detrimental effects or endogenous neuroprotection depending on the cell type (Fig. 1a, b)23,32,34,47,48,49,50,51,52,53,54,55.
a Perinatal brain injury induced either by systemic infection and/or by hypoxic/ischaemic events triggers a variety of pathophysiological processes, i.e. endothelial (EC, red/orange), microglia (M, green) and astrocyte (A, brown/red) activation leading to neuronal (N) and oligodendrocyte (O) and progenitor cell (NPC/OPC: neuronal/oligodendrocyte progenitor cell) degeneration and impaired maturation of these precursor cells. Injury-induced activation of EC but also peripheral immune cell (purple) activation (stars) facilitate leucocyte infiltration into the injured brain (swung arrows) via concerted bidirectional molecular interactions involving selectins, integrins and chemokines. However, interactions between leucocytes and ECs also contribute to vascular inflammation and damage to the blood brain barrier composed of ECs connected by tight junction (TJ) proteins, normally tight packed basal membranes (BM) and astrocytic endfeet. Besides contributing to endothelial damage, peripheral leucocytes are supposed to act from the perivascular cuffs by the release of proteolytic enzymes accelerating access of peripheral immune cells to the injured CNS parenchyma, which is further supported by release of chemotactic molecules of activated astrocytes and microglia. Within the CNS, an intense interaction between infiltrated leucocytes and CNS-resident cells leads to the release of a variety of pro-inflammatory and neurotoxic molecules by all involved cell types (triangles). The exact cell source, the time course of expression for each cell type and the relevance for the evolution of brain injury warrants further investigation. So far, only few and very ubiquitous detrimental mechanisms have been proposed as effectors of peripheral immune cells, i.e. neutrophil extracellular trap (NET) formation, reactive oxygen species (ROS) production, increased activity of inflammatory and basal membrane degrading enzymes (e.g. COX-2 and MMPs), and release of pro-inflammatory cytokines. These detrimental effects were specifically ascribed to neutrophils, pro-inflammatory M1 monocytes/macrophages, natural killer (NK) cells and subsets of T cells (i.e. γδ T cells and CD4 Th17 cells). b Emerging evidence supports a divergent role of different leucocyte subsets not only promoting damage but also contributing to resolution of inflammation/injury and mediating protection and/or promoting repair. Proposed mechanisms include the release of anti-inflammatory cytokines and growth factors by, for example, regulatory T and B cells as well as protective myeloid cells (e.g. M2 polarized macrophages and CCR2+ monocytes). However, most of these hypotheses are based on data derived from adult brain injury models. The contribution of peripheral immune cells to protection and repair in a time-dependent manner following the initial insult is still unclear (question marks). Furthermore, it is important to note that peripheral immune cell subsets have not only been supposed to contribute to repair and regenerative processes but also to be essential for endogenous neurodevelopment, i.e., to support oligodendrogenesis and microglia development. Whether these mechanisms take place from the periphery or the perivascular areas (e.g. meninges and/or CP) and how these signals are mediated is still unclear (dashed arrows with question mark). c The general concept about neuroinflammatory/degenerative but possibly also reparative processes mediated by peripheral leucocytes is further challenged by well described sex differences in neurodevelopmental outcome and inflammatory processes. The often-reported increased risk and worse outcome for males is associated with increased innate immune responses in males, supported by the fact that only male mice are protected after depletion of peripheral myeloid cells. Whether such sex dichotomy also applies to cells of the adaptive immune system is still unclear. d Another major aspect to be considered according to the hypothesized processes in (a) is the maturational stage, since many of them (full arrows with question marks) are still derived from preclinical research in adult brain injury models. However, the immune system and the brain reveal different responses over the life span, reflected by a rather immunoregulatory response in the neonatal stage to limit excess inflammation and an increased capacity of CNS regeneration despite of increased vulnerability of the developing brain. Therefore, concepts cannot be translated unequivocally from the adult organism to the neonatal system. Further challenges, which need to be taken into account for the predicted mechanisms shown in (a), are different immune and brain developmental stages in preterm compared to term newborns.
Neutrophils
The role of neutrophils in perinatal brain injury is poorly understood. Previous reports suggested rare extravasation of neonatal neutrophils into the injured brain, leading to the assumption that neutrophils are clinically less relevant47,56,57,58. However, recent work, including our own, revealed a significant accumulation of neutrophils in the parenchyma of HI-injured brain tissues50,52, which was preceded by an acute increase in circulating neutrophils50. In support of this, peripheral neutrophilia was observed in the first hours of life after NE in term infants, which was associated with poor neurodevelopmental outcome59. Furthermore, clinical evaluations with regard to neutrophil function and phenotype recently demonstrated that neutrophils of infants with NE display a dysregulated inflammatory response, revealed by a hyporesponsiveness to an additional ex vivo inflammatory stimulus, compared to healthy infants60, indicating a pre-activated state of neutrophils in NE infants. This is supported by recent analyses of serum and CSF biomarkers associated with activated neutrophils (e.g. interleukin-8 (IL-8)), which were upregulated in moderate or severe NE61. While these studies are of utmost importance for prediction of outcome, neutrophil function and phenotype in the brain can hardly be assessed clinically.
Experimentally it was shown that the early increase in the blood was followed by a rapid decline, when neutrophil numbers peaked in the brain50, suggesting migration of neutrophils into the brain. Nevertheless, their function in the brain is still a matter of debate. Previous work in models of HI and inflammation-sensitized HI revealed that neutrophil depletion before, but not after, HI is neuroprotective55,62, while a recent study demonstrated that depletion, initiated 12 h after the insult provides acute neuroprotection50. Several reasons may account for the discrepancy of study results, i.e. differences in outcome measures, methods to evaluate depletion efficiency, species and strategies of depletion. A further, so far underappreciated, aspect is the emerging concept of neutrophil plasticity and heterogeneity. Brain-infiltrated neutrophils reveal an enhanced activation, demonstrated by an increased reactive oxygen species (ROS) production. Furthermore, the frequency of overactivated/aged neutrophils, recently shown to contribute to vascular inflammation and myocardial infarction63, was elevated in brain-infiltrated neutrophils compared to blood-derived neutrophils in experimental HI-induced brain injury50. Signals that lead to these pronounced changes in neutrophils after entering the brain remain to be elucidated in future studies.
Different effector mechanisms of neutrophils have been suggested in experimental models for neonatal and adult ischaemic brain injury, e.g., formation of neutrophil extracellular traps and disruption of blood brain barrier (BBB) integrity by proteases that degrade basement membranes (e.g. matrix metalloproteinase 9) leading to accumulation of peripheral leucocytes55,56,64,65,66,67. Previous reports in adult and neonatal ischaemic stroke models as well as inflammation-sensitized HI revealed a pronounced accumulation of neutrophils in the vasculature and the perivascular space55,56,64,66, supporting that neutrophils contribute to vascular injury (Fig. 1a). In a model of neonatal stroke, it was further shown that peripheral neutralization of the neutrophil-specific chemokine CINC-1 leads to neutrophil adhesion, which was associated with adverse effects on BBB integrity56. Nevertheless, as stated before, a significant amount of neutrophils is also found in the brain parenchyma after hypoxic/ischaemic injury in adult as well as neonatal rodents, suggesting that neutrophils also act from the brain parenchyma behind the BBB and glia limitants (Fig. 1a)50,52,68. For example, neuroprotection after neutrophil depletion in neonatal HI was associated with reduced glial activation50, indicating that neutrophils promote glial activation thereby contributing to HI-induced neurodegeneration. However, interactions between neutrophils and glial cells are only poorly understood in adult as well as neonatal ischaemic brain injury69.
Neutrophils were also the first invaders in the context of the systemic inflammation in the absence of HI, which however depended on the kind of inflammatory stimulus34. In spite of similar marked responses in the periphery, activation of TLR2 but not TLR4 signalling was associated with a profound cerebral infiltration of peripheral immune cells, related to release of different leucocyte recruiting chemoattractants34. The functional role of neutrophils and their effector mechanisms in models of systemic inflammation-induced perinatal brain injury, regardless of stimulus, is still unknown.
Monocytes/macrophages
The second major cell population infiltrating the injured perinatal brain are monocytes and macrophages as shown in models of neonatal HI and systemic inflammation, induced by TLR2 activation34,44,48,49. Across all disease paradigms, injury-induced monocyte and macrophage infiltration was largely ascribed to upregulation of several monocyte-related chemoattractants (e.g. MCP-1)23,34,49. Nevertheless, while a pronounced infiltration of peripheral myeloid cells has been detected in neonatal HI48,52, monocyte infiltration was low and the majority of macrophages in acutely injured regions have been identified as microglia in a model of neonatal stroke70. Besides differences in models and species, a major reason for these discrepant study results, might be the difficulty to distinguish blood-derived from resident myeloid cells due to shared cell surface epitopes. A commonly used animal model to differentiate between both cell types is the CX3CR1GFP/+CCR2RFP/+ double transgenic mouse model, in which blood-derived CCR2+ monocytes are supposed to express the red fluorescent protein RFP and CX3CR1+ resident microglia the green fluorescent protein GFP71. Using these mice it was recently shown that neonatal HI leads to infiltration of CCR2+ monocytes accompanied by proliferation of CX3CR1+ resident microglia72. However, this mouse model has its limitations, since CX3CR1 is also expressed on peripheral immune cells (e.g. NK cells). Furthermore, infiltrating CCR2+ monocytes downregulate CCR2 after in situ reprogramming as reported in a model of sterile liver injury73. Therefore, an elegant transgenic model was recently developed, enabling fate mapping of peripherally derived CCR2 monocytes for at least 62 days after neonatal stroke, uncovering an underappreciated level of long-term monocyte-to-microglia transition after neonatal stroke74. Nevertheless, the full extent and spatial distribution of monocyte-derived microglia during long-term brain development are yet to be determined. Furthermore, it remains unclear, whether there is a subacute wave of invading monocytes after neonatal stroke that may be important for resolution of inflammation (Fig. 1b). This is an important question, considering previous reports in adult ischaemic brain injury, where selective targeting of CCR2 in bone marrow‐derived cells and peripheral monocytes caused delayed clinical deterioration, reduced regenerative angiogenesis and impaired long-term behavioural performance75,76. Potentially protective properties of CCR2+ monocytes were also identified in neonatal rodents exposed to hypoxia–ischaemia at PND3. HI in male CCR2 knockout but not in wild-type mice led to long-term spatial learning deficits associated with reduced numbers of activated macrophages/microglia in the damaged hippocampus77.
In addition to the difficulty of discrimination between peripheral and brain-resident myeloid cells, another level of complexity is added by the proposed concept of monocyte/macrophage heterogeneity. Classically activated M1 cells are supposed to promote neurodegeneration and alternatively M2 cells suggested to have anti-inflammatory and regenerative functions (Fig. 1b)78. Though, this myeloid cell dichotomy could be verified in vitro79, this concept seems to be too simple for the in vivo situation, as previously shown in experimental models of neonatal HI-induced brain injury80,81. Despite of the elegant possibility to perform selective analysis on ex vivo sorted myeloid cells from injured brains in different perinatal brain injury models, including traumatic brain injury, HI and perinatal stroke28,80,82,83,84, effects of peripheral monocytes/macrophages can hardly be distinguished from effects of resident microglia. Even though fluorescent-activated cell sorting (FACS)-based techniques may offer alternative strategies to separate both subsets (different CD45 expression), single-cell transcriptomic analyses may offer novel possibilities to identify specialized myeloid cell subsets. Besides cellular characterization and fate mapping, interventional approaches to selectively analyse the functional relevance of either peripheral or resident myeloid cells remain a major hurdle in neonatal research. While in adult brain injury models, the specific role of brain-resident microglia was mainly assessed by pharmacological inhibition of the colony-stimulating factor 1 receptor (CSF1R)85,86,87, this is not applicable in neonates due to long treatment periods needed. However, an elegant transgenic mouse model was developed, enabling rapid depletion of microglia, which was associated with aggravated HI-induced brain injury88. With regard to peripheral monocytes and macrophages, chlodronate liposomes might provide an efficient and selective strategy to deplete peripheral myeloid cells86, which needs further investigation in neonatal models.
Lymphoid cells of the innate immune system
Natural killer (NK) cells and gamma delta (γδ) T cells are increasingly recognized as potential modulators of neonatal brain injury. Clinical evaluations in NE demonstrated higher γδ T cell numbers and frequencies in neonates with NE and children with cerebral palsy compared to age-matched controls89. Furthermore, NK and γδ T cells from NE neonates revealed a more rapid inflammatory cytokine response upon ex vivo stimulation, suggesting that they were previously primed or activated. Interestingly, these alterations persisted into school age89.
NK cells
Experimentally, it was shown that NE caused by HI leads to pronounced infiltrations of NK cells even exceeding those of T cells46. Furthermore, increased brain injury after pharmacological T cell depletion was associated with a 3.5-fold increase of NK cells in HI-injured brain tissues48. However, data on the functional role of NK cells in neonatal brain injury are limited. HI-induced increases of the pro-inflammatory mediator cyclooxygenase 2 (COX-2) were diminished after interfering with NK cells by knocking down CD161, suggesting a pro-inflammatory role of NK cells in HI-induced brain inflammation and associated injury (Fig. 1a)46. While a causal relationship is still missing in neonates, NK cell ablation experiments in an adult brain ischaemia model demonstrated increased brain injury in NK cell-depleted mice, which was independent of T, NKT and B cells90. As a potential driver of detrimental effects recent work suggested that astrocytic IL-15 could aggravate post-ischaemic brain damage via propagation of NK cell-mediated immunity91.
While depletion of NK cells significantly ameliorated depression-like behaviour in adult LPS-treated mice, which was related to the chemotactic role of NK cells to recruit neutrophils92, the role of this subset in systemic inflammation-induced perinatal brain injury is unknown.
γδ T cells
Large numbers of innate γδ T cells were detected in post-mortem brain tissues of preterm human infants and in tissues of small and large animal models of perinatal HI93. Depletion of γδ T cells provided neuroprotection of HI-induced brain injury in preterm-equivalent rodents93, suggesting a detrimental role of this immune cell subset, which is supported by findings from adult injury models94. IL-17 released by γδ T cells is supposed to be the major detrimental effector molecule, activating metalloproteinases and inducing expression of pro-inflammatory and neutrophil-attracting cytokines (Fig. 1a)94. Therefore, modulating the activity of γδ T cells in perinatal brain injury might represent a novel therapeutic target. Intriguingly, antibiotic treatment-induced interstitial dysbiosis led to a reduction of meningeal IL-17+ γδ T cells associated with reduced ischaemic injury in an adult brain ischaemia model, uncovering a previously unrecognized role of these cells in the gut–brain axis95. The role of the neonatal microbiome for neurodevelopment is increasingly acknowledged96. The causal relationship between remote immune responses in the intestine and the development of perinatal brain injury is not well understood. However, the postnatal period is particularly critical for the development of microbiota composition and immune homoeostasis97,98. For instance, a TLR5-dependent regulatory circuit of neonatal bacterial colonization acting solely during the early neonatal period was recently reported to influences life-long microbiota composition99. Future research has to clarify the impact of microbiome-associated changes in the peripheral immune system on the development of perinatal brain injury.
With regard to systemic inflammation, it was shown that this innate T cell subset but not adaptive αβ T cells contribute to sepsis-induced white matter injury and subsequent motor function abnormalities in early life100.
Lymphoid cells of the adaptive immune system
Emerging evidence of clinical and preclinical studies indicate an important role of peripheral T and B cells in the development of perinatal brain injury44,46,47,48,53,54,101,102,103,104. The suggested dynamics of T and B cell infiltration observed at 1 and 7 days and an additional emergence three months after injury47,53,101,103 make them amenable to therapeutic intervention. This is supported by previous work in mature lymphocyte-deficient Rag1−/− mice that were protected from HI-induced white matter loss in 5-day-old mice103. However, a very recent report using severe combined immunodeficient (SCID) mice, which also lack functional T and B lymphocytes, did not observe differences in HI-induced brain injury in 12-day-old animals105. This discrepancy might be ascribed to different transgenic mouse strains, but most likely also to different developmental stages. Nevertheless, common to both studies is the lack of both, T and B cells, impeding clear conclusions about the specific contribution of each cell type.
T cells
To dissect the specific role of T cells adoptive transfer experiments with T and B cells in lymphocyte-deficient animal models are needed, as recently demonstrated in a model of adult stroke, where transfer of T cells but not B cells exacerbated infarction in Rag−/− mice106,107. However, adoptive transfer experiments via the intravenous route are challenging in neonatal animals. An alternative approach is the pharmacological modulation of systemic lymphoid cell subsets, e.g., by Fingolimod (FTY720), which is an immunomodulatory sphingosin-1-phosphate analogue, blocking egress of lymphocytes from lymphoid organs leading to reduced lymphocyte counts in the circulation and thus in the injured brain108,109. Own previous experimental work in neonatal rodents revealed that FTY720 particularly reduces circulating T cells but not B cells48. Interestingly, FTY720 treatment of HI-injured mice aggravated brain lesions48. These results suggest an endogenous protective function of neonatal T cells in HI-induced perinatal brain injury. Indeed, antibody-mediated T cell depletion also enhanced brain injury in our 9-day-old mouse model48. Furthermore, FTY720 blocked the selective endogenous infiltration of immunosuppressive/regulatory T cells (Treg)48, which are supposed to confer endogenous neuroprotection in adult brain injury (Fig. 1b)110. Therefore, the particular lack of neuroprotective Tregs upon FTY720 treatment may account for increased brain injury after depletion of the total T cell population. This is supported by previous work in an ovine preterm global HI model reporting that mesenchymal stem cells (MSCs) for neuroprotection induce peripheral T cell tolerance111.
The mode of action of T cells apparently depends on the kind of injurious stimulus, since it was previously shown that in contrast to ischaemic brain injury the general lack of αβ T cells does not influence LPS-induced white matter loss100. However, pro-inflammatory αβ T cell subtypes, i.e., CD4 Th17 cells are supposed to play an important role in the combined setting of peripheral inflammation and HI, demonstrated by an increased systemic frequency of pro-inflammatory Th17 cells compared to healthy controls54.
B cells
Little is known about B cells. Neonatal HI leads to an upregulation of genes involved in B cell proliferation and differentiation112. Previous work further revealed that enhanced HI-induced brain injury in adenosine A1 receptor knockout mice was associated with reduced activation and infiltration of IL-10-producing B lymphocytes, indicating a protective function of these cells in this setting104. In support of this it was shown that regulatory B cells control inflammation in neonatal mice via production of IL-10 following TLR9 stimulation113.
Infiltration routes of peripheral immune cells
Blood brain barrier
Different barrier systems have been described to protect the brain against the invasion of harmful pathogens and leucocytes, thereby contributing to the so called “immune privilege of the brain”. Traditionally, these barriers were supposed to be immature during development, contributing to increased vulnerability of the immature brain. However, emerging evidence suggests that these barriers are well developed to combat injurious insults114. In models of neonatal stroke, it was reported that blood brain barrier (BBB) integrity is more preserved in neonates compared to adults56. However, common to all perinatal insults, discussed in this review, is a general disturbance of BBB integrity compared to the healthy brain, as excellently reviewed by Mallard et al.114. Even though the detailed underlying molecular and cellular mechanisms are only partially understood, increased BBB injury is associated with an increased accumulation of peripheral immune cells in the injured brain. Suggested mechanisms involve damage to tight junction proteins115,116, upregulation of chemokines23,117 and endothelial adhesion molecules118,119,120 and disruption of the normally tight packed basal membranes50 (Fig. 1a).
Blood–CSF barrier
In addition to the traditional infiltration route through the impaired BBB, further anatomical interfaces as potential gateways to the brain are increasingly recognized in adult and neonatal brain injury. These include the choroid plexus (CP) through CSF and the subarachnoid space through meningeal vessels121,122,123. Recently the CP was suggested to be an important infiltration route of monocytes and neutrophils in the context of neonatal stroke, supposed to be mediated through CX3CR1-CCR2-dependent mechanisms124. In the context of systemic perinatal inflammation, it was shown that TLR2 activation leads to strong neutrophil and monocyte infiltration to the CSF through the CP associated with TLR2-specific alterations of chemotactic molecule expression and basement membrane remodelling33,34. First therapeutic approaches have been tested, i.e. N-acetylcysteine blocked neutrophil migration across the endothelium of choroidal stromal vessels and the epithelium, both forming the blood–CSF barrier117.
Alternative routes
Further alternative infiltration routes have been suggested in adult brain injury models. A recently discovered route for neutrophil migration into the ischaemic brain is displayed by direct connections from the skull bone marrow to the brain125,126. However, the presence of these structures in the neonatal skull has not been investigated. Other potential gateways may be lymphatic vessels in the meninges127, recently suggested to be used for drainage of CSF components and lymphocyte migration to the draining lymph nodes during chronic neuroinflammation128. Whether and how these alternative infiltration routes and immunological communication hubs orchestrate the inflammatory response in the injured neonatal brain is still unknown. Further research in this direction may uncover new possibilities for selective intervention.
Impact of sex
Male sex is a well-established epidemiological risk factor for poor neurodevelopmental outcome and increased innate neuroinflammatory responses after perinatal brain injury (Fig. 1c)129,130,131,132,133,134. The risk for perinatal stroke or to experience an HI insult is supposed to be higher in males combined with more severe neurological deficits133,135,136. Furthermore, clinical and experimental studies suggested a sexual dimorphism in response to neuroprotective therapies, e.g. hypothermia130,133,137. Infections, both during pregnancy and in childhood also contribute to the sex-dependent risk for the development of long-term neurological sequelae, specifically for neuropsychiatric disorders138.
Preclinical studies demonstrated an increased HI-induced brain atrophy in males139, associated with differences in long-lasting immune responses, revealed by elevated circulating levels of tumour necrosis factor (TNF)-α in adolescent male mice and a more pronounced lymphocyte infiltration in injured brain hemispheres accompanied by reduced neurogenesis129. Interestingly, sex differences become particularly evident in the phase of secondary neurodegeneration and inflammation. This is supported by Mriza et al.132, who observed more severe damage and increased pro-inflammatory cytokine expression in brain tissues of males 3 days but not 1 day after HI. Similarly, males exhibited larger tissue loss than females 3 months after experimental neonatal stroke, but infarct volumes did not differ 3 days after ischaemia84,140. With regard to systemic inflammation, a similar sex dichotomy was described, i.e., infection with Staphylococcus epidermis, activating TLR2, potentiates HI-induced brain injury in male but not in female animals31. There are, however, first indications, that activation of TLR3 with the viral mimetic Poly IC increases early extrinsic apoptotic pathways in neonatal female but not in male rodents, though males revealed increased inflammatory responses (i.e. astrogliosis and IL-6 expression) in the brain141.
Sex differences in cell death pathways and inflammatory responses have been discussed130, but their origins in perinatal injury are yet poorly understood. While a large body of evidence points to an important role of microglia84,138,142,143, the impact of sex on peripheral leucocytes is not well explored. This is, however, important, considering pronounced physiological differences between neonatal male and female immune responses, reflected by more robust innate immune responses in males, while females have higher CD4/CD8 T cell ratios144. A major driver of the well-known sex differences in immunity are sex hormones with a plethora of effects on immune system function144. For example, oestrogens promote the expansion of Tregs and induce Th2 responses145, accompanied by a decreased production of IL-17 by pro-inflammatory Th17 cells146. Even though, hormonal influences on neonatal immune cells and consequently on outcome after perinatal brain injury cannot be entirely excluded, lacking sex-hormone differences before puberty indicate intrinsic/genetic differences132,144. Preclinical reports demonstrating no sex differences in hormone levels but differences in inflammatory responses and outcome129,132 support that intrinsic mechanisms may account for divergent susceptibility to the development of neurological deficits. For example, first preclinical studies revealed an increased myeloid cell infiltration in males following neonatal stroke84. Furthermore, antibody-mediated depletion of peripheral myeloid cells provided neuroprotection only in male mice52. The impact of sex on cells of the adaptive immune system in perinatal brain injury is largely unknown. Mirza et al.132 showed an increased infiltration of lymphocytes in HI-injured brains of males. However, cells types were not discriminated. Considering the complex and divergent functions of different lymphocyte subtypes (see sections “Lymphoid cells of the innate immune system” and “Lymphoid cells of the adaptive immune system”, Fig. 1a, b), further in-depth analyses of specific cell types are needed. Potentially important cells might be regulatory T cells (Treg), for which sex-dependent differences in functionality and phenotype have been reported in different adult disease paradigms144. Besides sex-hormone-dependent mechanisms, differences in the transcriptomic and metabolic profile between female and male Tregs have been recently discussed in the context of chronic intestine and visceral adipose tissue inflammation147,148. There are no such studies in adult stroke models available since only male mice were used in the majority of studies. In the context of perinatal brain injury, studies on sex-specific Treg responses are also pending.
In view of the huge amount of epidemiological evidence over more than two decades, preclinical in vivo research is now beginning to include sex-stratified analyses across a huge variety of perinatal injury models. However, our understanding of potential functional and phenotypical sex differences in cells of the peripheral immune system and their impact on the development of perinatal brain injury is still in its infancy.
Impact of maturational state
The uniqueness of the neonatal immune system is demonstrated by significant differences in quantity and function of innate and adaptive immune cells compared to adults (Fig. 1d), as comprehensively summarized by Tsafaras et al.41. While the impact of these pronounced differences is increasingly acknowledged in immunological research, including sepsis and vaccination research, their impact on neurodevelopmental disorders is still in its infancy. This is, however, of utmost importance, since the brain is still developing, which makes it more vulnerable to noxious stimuli on the one hand. On the other hand, the maturing brain is supposed to have an increased regenerative capacity, offering unappreciated opportunities for intervention, which can hardly be derived from research in adult brain injury (Fig. 1d). Another level of complexity is added by marked differences in immune responses and brain injury mechanisms between injury affecting preterm and term-born babies (Fig. 1d). So far, systematic comparative analyses for each of the discussed leucocyte subsets with regard to different immune cell functions comparing either perinatal vs. adult brain injury or preterm vs. term-born are rare. Therefore, we will focus on two main subsets of immune cells in this chapter, i.e., neutrophils and T cells.
Neutrophils
Even though neonatal neutrophils are believed to be immature and impaired in function compared to adult neutrophils (e.g. weaker responses to infection, less migratory capacity, less ROS production)149,150,151, they infiltrate the injured brain early after neonatal HI50,52. Similar detrimental effector mechanisms appear to be involved in adult and neonatal ischaemic brain injury, contributing to injury evolvement in the early disease phase, e.g., through ROS production50 and interaction with the vasculature14,55,56 (Fig. 1a). Nevertheless, the impact of neutrophils also seems to depend on the maturational stage in the neonatal phase. For instance, in rodents with inflammation-sensitized HI-induced brain injury, an insult at PND1 (corresponding to preterm birth) did not lead to accumulation of neutrophils, while pronounced neutrophil infiltration was observed in the same experimental setting, when induced at PND12 (corresponding to term birth)23. These age-dependent differences correlated with significant and longer-lasting cerebral expression of the neutrophil-specific chemokine CINC-1 in term-born equivalent rodents23.
In addition to timing of the insult, neutrophils may have a divergent role in different time frames after the initial insult. Until now, the majority of adult and neonatal studies focused on the role of neutrophils in the acute injury phase, neglecting effects on endogenous repair mechanisms occurring days to weeks later50,52,55,62,107,152. Recent experimental findings showed a second infiltration of total myeloid cells 7 days after HI52,53, when regeneration and repair are initiated153. Considering that perinatal brain injury induced inflammatory processes last into the secondary and tertiary disease phase154 overlapping with endogenously induced regenerative processes, myeloid cells might contribute to endogenous repair mechanisms (Fig. 1b), e.g., be the release of growth factors and proangiogenic factors, as recently described in adult brain injury models155,156,157,158,159. In support of this, it was recently shown that neutrophils promote regeneration in an adult model of optic nerve regeneration and spinal cord injury159.
T cells
In contrast to adult ischaemic brain injury160, peripheral lymphocyte and specifically T cell depletion by FTY720 treatment in HI-injured mice aggravated brain lesions48. These results do not only confirm previous reports about questionable translation from adults to neonates10, but also suggest an endogenous protective function of neonatal T cells in HI-induced perinatal brain injury. Compared to adults, neonatal T cells reveal an increased bias towards anti-inflammatory T cell subsets40, which might provide an endogenous protective mechanism to limit excess inflammation in the neonatal organism. The selective blockade of endogenous infiltration of Tregs in FTY720-treated animals48 may explain differences in outcomes between neonatal and adult mice. This is supported by the fact that neonatal Tregs are supposed to have an increased activation state and immunosuppressive function compared to adults161,162,163. Furthermore, T cell function also seems to depend on the maturational stage already in the neonatal phase. Studies indicating a detrimental role of T cells preferentially investigated preterm-equivalent HI models using PND4 and PND7 rodents100,103 or transient umbilical cord occlusion111. In contrast, analyses in term-born equivalent PND9 and PND12 mice, either lacking both, T and B cells, or with selective T cell depletion showed no differences or indicated endogenous neuroprotection by T cells, respectively48,105. Taking into account that the stage of white matter development and axonal outgrowth in the rodent CNS between PND1 and PND7 corresponds to 23–36 weeks gestation in human brain development164, infiltrated T cells may impede vulnerable CNS maturation processes in this critical period165. In contrast, in the term-equivalent brain of PND9-PND12 rodents, T cells may rather protect from HI-induced damage of mature neural cells. Physiological changes in the peripheral immune system between PND5 and PND9 rodents revealed by a continuous increase of lymphocytes and a decrease of neutrophils within the first 2 weeks of life may also play a role44,166. In spite of lacking systematic ontogenetic characterization of T cell subpopulations, first clinical evaluations demonstrated that extreme prematurity is associated with increased Treg frequencies and suppressive activity compared to Tregs of term neonates167,168.
Similar to neutrophils, T cells and their subsets may obtain different functions in different time frames after the initial insult. Experimental studies showed that there is a second wave of T cell infiltration 7 days after HI53,101, which might be important for endogenous repair and neurogenesis on the one hand. On the other hand, the second accumulation of CD4 T cells 7 days after HI was also associated with enhanced expression of RORγt, a transcription factor specific for the pro-inflammatory CD4 Th17 T cell subset101. The contribution of different T cell subpopulations to tertiary mechanisms of perinatal brain injury is still unknown and warrants further investigation.
Taken together, further clinical and experimental studies are needed to dissect the role of the different leucocyte subsets in different time frames after injury but also for different timings of the insult (i.e. preterm vs. term). This would allow definition of selective therapeutic windows for modulation of specific leucocyte subsets to enable protection, repair, and restoration of physiological brain development.
Implications for treatment
First clinical evaluations and experimental intervention studies identified the peripheral immune system as possible target for novel therapeutic strategies. The detrimental role of early neutrophils and myeloid cells was uncovered by selective depletion of these cell types50,52, which however bears severe risks for neonates and is therefore no therapeutic option. According to previous studies in adult stroke models and perinatal brain injury caused by intrauterine chorioamnionitis, an alternative strategy might be the inhibition of peripheral leucocyte infiltration, e.g., by blockade of adhesion molecules and chemokine receptors48,107,169. However, a better understanding of the increasingly recognized plasticity and heterogeneity of myeloid cell types may provide new opportunities to modulate immune cells in the periphery towards protective cell types, as previously suggested in models of optic nerve crush, spinal cord injury and stroke155,159.
Hypothermia and immunomodulation
Neuroprotection induced by delayed neutrophil depletion 12 h after injury suppose larger therapeutic windows of immunomodulatory therapies compared to standard therapies like hypothermia (HT). Therefore, interfering with peripheral immune responses may overcome current limitations of HT. However, potential interaction effects between anti-inflammatory HT and immunomodulatory therapies should be considered. In the clinical setting HT induces a transient leukopenia between 60 and 72 h and persisting low white blood cell counts after rewarming were associated with poor outcome170. Preclinical data systematically investigating the impact of HT on the fate and function of different leucocyte subsets is still missing. Even though recent work with SCID mice lacking T and B cells did not indicate any adverse interaction effects105, further work is needed to evaluate either interaction or potential synergistic effects between HT and immunomodulatory therapies.
Impact of disease aetiology and time point of intervention
Post-hypoxic neutrophil depletion provided acute neuroprotection in our work in a mouse model of pure HI50, while being less efficient in LPS-sensitized HI55. Furthermore, FTY720 had detrimental effects in pure HI48, while being protective in inflammation-sensitized HI54. Discrepant study results may be related to different injury severities, i.e. animals in the combined setting revealed up to 40% tissue loss in the cortex while only 10% cortical tissue loss were observed in pure HI48,54. Injury severity seems particularly important for the extent of neuroinflammatory responses but also for the efficiency of immunomodulatory therapies, as already reported in adult models of ischaemic stroke at the example of FTY720 treatment160,171,172,173. Besides different injury severities, disease aetiology might play a major role in this context, since systemic activation of the immune system by LPS strongly alters the peripheral immune system, as previously shown34. Therefore, immune cell function in the HI-injured brain most likely differs compared to sterile inflammation induced by the sole HI event. However, a systematic comparison of leucocyte subset function in the brain between both entities is still lacking. In addition to the experimental setting and disease aetiology, the time point of intervention might affect treatment outcome. To date, the majority of studies focused on the acute role of peripheral immune cells, e.g., by initiation of cell depletion prior to or shortly after HI. While these approaches target the initial influx of cells, the relevance of a second infiltration peak 7 days after HI observed for myeloid cells and T cells52,101 can hardly be predicted by these experimental approaches. Furthermore, transgenic mouse models (Rag−/−, SCID, αβ T cell receptor−/−, γδ T cell receptor−/−) have a permanent depletion of specific T cell subsets or of both, T and B cells93,100,103,105. In the majority of these studies, analyses were performed 7 days after the insult. Therefore, modulation of the early, the late or of both infiltration time points might have led to the observed study results. The same holds true for a single FTY720 application shortly after HI, which led to a sustained depletion for at least for 7 days48. Therefore, further studies, selectively targeting distinct time points after the insult will be important, considering the different time windows of opportunity.
Immunomodulatory effects of stem-cell based regenerative therapies
In addition to selectively targeting cells of the peripheral immune system, multimodal therapies, indirectly modulating peripheral leucocytes, i.e., by MSC and MSC-derived extracellular vesicles (EV) represent promising therapeutic approaches. In a variety of classically immunological but also other disease paradigms, MSCs have been described to modulate innate and adaptive immune responses, thereby reducing tissue injury and creating an environment supporting tissue repair and regeneration174,175. With regard to perinatal brain injury, a tremendous amount of reports revealed the neuroprotective potential of MSCs and MSC-EVs19,81,111,119,176,177,178,179,180,181,182,183. However, the underlying mode of action of these stem-cell-based regenerative therapies are poorly understood. While the majority of studies particularly focused on cell damaging and regenerative effects in the brain, MSCs’ and MSC-EVs’ immunomodulatory effects in the periphery have been rarely addressed in perinatal brain injury models. Intravenous MSC administration in an ovine global HI model revealed that protective effects were associated with a diminished HI-induced splenic involution, increased T cell tolerance and reduced cerebral T cell infiltration111,177. These studies suggest that modulation of peripheral immune cells may contribute to stem-cell-based protective and regenerative effects. With regard to clinical translation, administration routes have been adapted to intranasal (i.n.) applications of MSCs and MSC-EVs, particularly in rodent models119,176,182. While this administration route provides similar protective effects, the mode and place of action may differ. Even though it is supposed that i.n. application of MSCs and MSC-EVs reach the brain easier and may rather directly modulate injurious and regenerative processes in the brain176,184,185,186, the potential impact on the periphery via this route is not well explored. However, first analyses of serum samples from rodents treated with MSCs via the intranasal route indicate that i.n. administration of stem cells may also modulate peripheral immune responses119. A major challenge in this research field will be to dissect immediate targets in the brain and indirect mechanisms in the periphery to improve efficiency of stem-cell-based therapeutic approaches.
Conclusion and perspectives
The pathogenesis of perinatal brain injury is complex and multifactorial. In this review, we focused on brain injury induced by systemic inflammation and hypoxic/ischaemic insults in the perinatal period. Though different in aetiology, all of them are associated with neuroinflammatory processes involving the peripheral immune system. Against the common concept of an immature and impaired immune system, an emerging body of evidence suggests a tightly regulated responsiveness of neonatal leucocyte subtypes to injurious stimuli associated with perinatal brain injury. Modulation of peripheral immune responses may offer novel opportunities for intervention due to easier access compared to the target organ, the brain. However, our knowledge on the contribution of different leucocyte subsets to acute neurodegeneration but also repair is too immature to propose novel therapeutic strategies. This will require further systematic analysis in the periphery and in the injured brain, experimentally and clinically. Experimentally, the majority of findings is based on studies in rodents, which need to be verified in large translational animal models and clinical studies. While clinical correlation analyses between peripheral immune responses and neurological outcome will provide utmost important predictive values, experimental studies may uncover cause-and-consequence interrelations.
A major challenge is to improve our understanding about the plasticity and heterogeneity of different leucocyte subsets depending on several factors, like sex, time window of assessment, tissue environment and aetiology of disease. Furthermore, time and place of immune cell priming needs to be elucidated. In addition to the discussed gut–brain axis, interactions between lung and brain may also shape leucocyte phenotype and function since the plethora of noxious stimuli associated with perinatal brain injury and long-term neurological deficits do not exclusively affect the brain. Therefore, a major need will be the identification of direct cellular targets of different leucocyte subtypes to answer the question, whether they act already in the periphery modulating other peripheral immune cells or whether they mediate their effects directly in the brain, e.g., through interaction with other CNS cells like microglia187. Moreover, in view of previous work, aiming to identify inflammatory biomarkers for prediction of outcome it needs to be elucidated to which extent peripheral immune activation is cause or consequence of perinatal brain injury. While increased levels of IL-6, C-reactive protein and myeloperoxidase in umbilical cord serum were associated with preterm birth, these did not correlate with poor neurodevelopmental outcomes188. However, increased IL-6 levels before initiation of HT in term-born infants with HIE were associated with adverse short-term MRI outcome189. With regard to peripheral cellular responses, it was suggested that, on the one hand, high neutrophil numbers after HIE are associated with poor outcome59. On the other hand, HT-induced reductions of white blood cell counts were also associated with worse outcome170. According to this, reversal of stroke-induced lymphopenia in a model of adult ischaemic brain injury was associated with enhanced neuroregeneration and functional improvement190. These data demonstrate the complexity of the topic and the urgent need for an in-depth understanding of the delicate balance between protective and detrimental peripheral immune responses. When interfering with the immune system, it should be taken into account that peripheral immune cells may not only promote tissue injury but also contribute to regeneration and physiological brain development (Fig. 1b), as elegantly shown at the example of CD4 T and B cell subsets191,192. Another aim of future work to answer the difficult question of cause and consequence will be to clearly distinguish the role of peripheral immune cells in (infectious) systemic inflammation-induced brain injury and sterile tissue inflammation, caused by ischaemic events. First preclinical data indicate that both insults may activate different inflammatory pathways. For example, systemic endotoxin exposure robustly induced TLR2 expression in the brain and increased levels of several inflammatory cytokines/chemokines, while TLR2 expression and inflammatory responses were reduced in the paradigm of sterile inflammation induced by intracerebral IL-1β injection or neonatal stroke49.
Another important challenge is the understanding of timing and consequences of leucocyte priming during the perinatal period for long-term neurodevelopment. In spite of the overwhelming data linking perinatal inflammation to long-term neurological impairment37,138,193, the majority of research focused on early and late cytokine responses and the important role of microglia. The impact of peripheral immune cell priming by noxious stimuli during the perinatal period on the development of long-term neurological disorders is only poorly understood. Recently, a causal link between increased neonatal monocyte infiltration caused by the combination of maternal inflammation and postnatal HI and development of autistic-like behaviours in the offspring was demonstrated194. Furthermore, experimental chorioamnionitis, a leading cause of preterm birth, resulted in increased cerebral neutrophil activation at PND7, which could be reversed by inhibition of early neutrophil infiltration leading to amelioration of grey and white matter injury at juvenile age (i.e. PND21)169.
Taken together, in spite of the high translational potential, the present review demonstrates that this topic is a novel research field, just beginning to develop. Both, detailed preclinical research, elucidating the causal interrelationship between the peripheral immune and the central nervous system and correlative clinical evaluations will help to improve our understanding of factors contributing to peripheral immune cell plasticity and function in a time-dependent manner. These upcoming findings may offer possibilities to develop novel therapies for treatment of perinatal brain injury and for prevention of long-term neurological disorders.
References
Graham, E. M., Ruis, K. A., Hartman, A. L., Northington, F. J. & Fox, H. E. A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am. J. Obstet. Gynecol. 199, 587–595 (2008).
Kurinczuk, J. J., White-Koning, M. & Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum. Dev. 86, 329–338 (2010).
Lawn, J., Shibuya, K. & Stein, C. No cry at birth: global estimates of intrapartum stillbirths and intrapartum-related neonatal deaths. Bull. World Health Organ. 83, 409–417 (2005).
Darmency-Stamboul, V. et al. Antenatal factors associated with perinatal arterial ischemic stroke. Stroke 43, 2307–2312 (2012).
Greenham, M. et al. Early predictors of psychosocial functioning 5 years after paediatric stroke. Dev. Med. Child Neurol. 59, 1034–1041 (2017).
Grunt, S. et al. Incidence and outcomes of symptomatic neonatal arterial ischemic stroke. Pediatrics 135, e1220–e1228 (2015).
Klemme, M. et al. Neonatal arterial ischemic stroke—a hospital based active surveillance study in Germany. Klin. Padiatr. 229, 142–146 (2017).
Rice, J. E. 3rd, Vannucci, R. C. & Brierley, J. B. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann. Neurol. 9, 131–141 (1981).
Derugin, N. et al. Evolution of brain injury after transient middle cerebral artery occlusion in neonatal rats. Stroke 31, 1752–1761 (2000).
Mallard, C. & Vexler, Z. S. Modeling ischemia in the immature brain: how translational are animal models? Stroke 46, 3006–3011 (2015).
Larpthaveesarp, A. & Gonzalez, F. F. Transient middle cerebral artery occlusion model of neonatal stroke in P10 rats. J. Vis. Exp. 122, e54830 (2017).
Patel, S. D. et al. Therapeutic hypothermia and hypoxia-ischemia in the term-equivalent neonatal rat: characterization of a translational preclinical model. Pediatr. Res. 78, 264–271 (2015).
Reinboth, B. S. et al. Endogenous hypothermic response to hypoxia reduces brain injury: implications for modeling hypoxic-ischemic encephalopathy and therapeutic hypothermia in neonatal mice. Exp. Neurol. 283, 264–275 (2016).
Fernandez-Lopez, D., Natarajan, N., Ashwal, S. & Vexler, Z. S. Mechanisms of perinatal arterial ischemic stroke. J. Cereb. Blood Flow. Metab. 34, 921–932 (2014).
Millar, L. J., Shi, L., Hoerder-Suabedissen, A. & Molnar, Z. Neonatal hypoxia ischaemia: mechanisms, models, and therapeutic challenges. Front. Cell Neurosci. 11, 78 (2017).
Kuban, K. C. et al. The breadth and type of systemic inflammation and the risk of adverse neurological outcomes in extremely low gestation newborns. Pediatr. Neurol. 52, 42–48 (2015).
Leviton, A. et al. The risk of neurodevelopmental disorders at age 10 years associated with blood concentrations of interleukins 4 and 10 during the first postnatal month of children born extremely preterm. Cytokine 110, 181–188 (2018).
Mitha, A. et al. Neonatal infection and 5-year neurodevelopmental outcome of very preterm infants. Pediatrics 132, e372–e380 (2013).
Drommelschmidt, K. et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate inflammation-induced preterm brain injury. Brain Behav. Immun. 60, 220–232 (2017).
Favrais, G. et al. Systemic inflammation disrupts the developmental program of white matter. Ann. Neurol. 70, 550–565 (2011).
Tann, C. J. et al. Perinatal risk factors for neonatal encephalopathy: an unmatched case-control study. Arch. Dis. Child Fetal Neonatal Ed. 103, F250–F256 (2018).
Jenster, M. et al. Maternal or neonatal infection: association with neonatal encephalopathy outcomes. Pediatr. Res. 76, 93–99 (2014).
Brochu, M. E., Girard, S., Lavoie, K. & Sebire, G. Developmental regulation of the neuroinflammatory responses to Lps and/or hypoxia-ischemia between preterm and term neonates: an experimental study. J. Neuroinflammation 8, 55 (2011).
Falck, M. et al. Neonatal systemic inflammation induces inflammatory reactions and brain apoptosis in a pathogen-specific manner. Neonatology 113, 212–220 (2018).
Fleiss, B. et al. Inflammation-induced sensitization of the brain in term infants. Dev. Med. Child Neurol. 57(Suppl 3), 17–28 (2015).
Martinello, K. A. et al. Acute Lps sensitization and continuous infusion exacerbates hypoxic brain injury in a piglet model of neonatal encephalopathy. Sci. Rep. 9, 10184 (2019).
Osredkar, D. et al. Hypothermia is not neuroprotective after infection-sensitized neonatal hypoxic-ischemic brain injury. Resuscitation 85, 567–572 (2014).
Serdar, M. et al. Early pro-inflammatory microglia activation after inflammation-sensitized hypoxic-ischemic brain injury in neonatal rats. Front. Cell Neurosci. 13, 237 (2019).
Wang, X. et al. Lipopolysaccharide sensitizes neonatal hypoxic-ischemic brain injury in a Myd88-dependent manner. J. Immunol. 183, 7471–7477 (2009).
Wood, T. et al. A ferret model of inflammation-sensitized late preterm hypoxic-ischemic brain injury. J. Vis. Exp. https://doi.org/10.3791/60131 (2019).
Gravina, G. et al. Staphylococcus epidermidis Sensitizes perinatal hypoxic-ischemic brain injury in male but not female mice. Front. Immunol. 11, 516 (2020).
Lai, J. C. Y. et al. Vancomycin is protective in a neonatal mouse model of Staphylococcus epidermidis-potentiated hypoxic-ischemic brain injury. Antimicrob. Agents Chemother. 64, e02003–19 (2020).
Mottahedin, A. et al. Systemic activation of toll-like receptor 2 suppresses mitochondrial respiration and exacerbates hypoxic-ischemic injury in the developing brain. J. Cereb. Blood Flow. Metab. 37, 1192–1198 (2017).
Mottahedin, A., Smith, P. L., Hagberg, H., Ek, C. J. & Mallard, C. Tlr2-mediated leukocyte trafficking to the developing brain. J. Leukoc. Biol. 101, 297–305 (2017).
Bi, D. et al. Staphylococcus epidermidis bacteremia induces brain injury in neonatal mice via Toll-like receptor 2-dependent and -independent pathways. J. Infect. Dis. 212, 1480–1490 (2015).
Fleiss, B. et al. Microglia-mediated neurodegeneration in perinatal brain injuries. Biomolecules 11, 99–120 (2021).
Hagberg, H., Gressens, P. & Mallard, C. Inflammation during fetal and neonatal life: implications for neurologic and neuropsychiatric disease in children and adults. Ann. Neurol. 71, 444–457 (2012).
Mallard, C., Tremblay, M. E. & Vexler, Z. S. Microglia and neonatal brain injury. Neuroscience 405, 68–76 (2019).
Pierre, W. C. et al. Neonatal microglia: the cornerstone of brain fate. Brain Behav. Immun. 59, 333–345 (2017).
Adkins, B., Leclerc, C. & Marshall-Clarke, S. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 4, 553–564 (2004).
Tsafaras, G. P., Ntontsi, P. & Xanthou, G. Advantages and limitations of the neonatal immune system. Front Pediatr. 8, 5 (2020).
Zhao, J. et al. Hyper innate responses in neonates lead to increased morbidity and mortality after infection. Proc. Natl Acad. Sci. USA 105, 7528–7533 (2008).
Ulas, T. et al. Corrigendum: S100-Alarmin-induced innate immune programming protects newborn infants from sepsis. Nat. Immunol. 18, 1173 (2017).
Lai, J. C. Y. et al. Immune responses in perinatal brain injury. Brain Behav. Immun. 63, 210–223 (2017).
Wang, Y., Zhang, J. H., Sheng, J. & Shao, A. Immunoreactive cells after cerebral ischemia. Front. Immunol. 10, 2781 (2019).
Fathali, N. et al. Splenic immune cells in experimental neonatal hypoxia-ischemia. Transl. Stroke Res. 4, 208–219 (2013).
Bona, E. et al. Chemokine and inflammatory cell response to hypoxia-ischemia in immature rats. Pediatr. Res. 45, 500–509 (1999).
Herz, J. et al. Peripheral T cell depletion by Fty720 exacerbates hypoxic-ischemic brain injury in neonatal mice. Front. Immunol. 9, 1696 (2018).
Lalancette-Hebert, M. et al. Live imaging of the innate immune response in neonates reveals differential Tlr2 dependent activation patterns in sterile inflammation and infection. Brain Behav. Immun. 65, 312–327 (2017).
Mulling, K. et al. Neutrophil dynamics, plasticity and function in acute neurodegeneration following neonatal hypoxia-ischemia. Brain Behav. Immun. 92, 234–244 (2021).
Shrivastava, K., Chertoff, M., Llovera, G., Recasens, M. & Acarin, L. Short and long-term analysis and comparison of neurodegeneration and inflammatory cell response in the ipsilateral and contralateral hemisphere of the neonatal mouse brain after hypoxia/ischemia. Neurol. Res. Int. 2012, 781512 (2012).
Smith, P. L. P. et al. Peripheral myeloid cells contribute to brain injury in male neonatal mice. J. Neuroinflammation 15, 301 (2018).
Winerdal, M. et al. Long lasting local and systemic inflammation after cerebral hypoxic ischemia in newborn mice. PLoS ONE 7, e36422 (2012).
Yang, D. et al. Blocking lymphocyte trafficking with Fty720 prevents inflammation-sensitized hypoxic-ischemic brain injury in newborns. J. Neurosci. 34, 16467–16481 (2014).
Yao, H. W. & Kuan, C. Y. Early neutrophil infiltration is critical for inflammation-sensitized hypoxic-ischemic brain injury in newborns. J. Cereb. Blood Flow. Metab. 40, 2188–2200 (2020).
Fernandez-Lopez, D. et al. Blood-brain barrier permeability is increased after acute adult stroke but not neonatal stroke in the rat. J. Neurosci. 32, 9588–9600 (2012).
Hudome, S. et al. The role of neutrophils in the production of hypoxic-ischemic brain injury in the neonatal rat. Pediatr. Res 41, 607–616 (1997).
Vexler, Z. S. & Yenari, M. A. Does inflammation after stroke affect the developing brain differently than adult brain? Dev. Neurosci. 31, 378–393 (2009).
Morkos, A. A. et al. Elevated total peripheral leukocyte count may identify risk for neurological disability in asphyxiated term neonates. J. Perinatol. 27, 365–370 (2007).
O’Dea, M. I. et al. Dysregulated monocyte and neutrophil functional phenotype in infants with neonatal encephalopathy requiring therapeutic hypothermia. Front. Pediatr. 8, 598724 (2020).
Sweetman, D. U. et al. Neonatal encephalopathy is associated with altered Il-8 and Gm-Csf which correlates with outcomes. Front. Pediatr. 8, 556216 (2020).
Palmer, C., Roberts, R. L. & Young, P. I. Timing of neutrophil depletion influences long-term neuroprotection in neonatal rat hypoxic-ischemic brain injury. Pediatr. Res. 55, 549–556 (2004).
Adrover, J. M. et al. A neutrophil timer coordinates immune defense and vascular protection. Immunity 50, 390–402 e310 (2019).
Enzmann, G. et al. The neurovascular unit as a selective barrier to polymorphonuclear granulocyte (Pmn) infiltration into the brain after ischemic injury. Acta Neuropathol. 125, 395–412 (2013).
Kang, L. et al. Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke. Nat. Commun. 11, 2488 (2020).
Perez-de-Puig, I. et al. Neutrophil recruitment to the brain in mouse and human ischemic stroke. Acta Neuropathol. 129, 239–257 (2015).
Yang, Y. & Rosenberg, G. A. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke 42, 3323–3328 (2011).
Otxoa-de-Amezaga, A. et al. Location of neutrophils in different compartments of the damaged mouse brain after severe ischemia/reperfusion. Stroke 50, 1548–1557 (2019).
Greenhalgh, A. D., David, S. & Bennett, F. C. Immune cell regulation of glia during cns injury and disease. Nat. Rev. Neurosci. 21, 139–152 (2020).
Denker, S. P. et al. Macrophages are comprised of resident brain microglia not infiltrating peripheral monocytes acutely after neonatal stroke. J. Neurochem. 100, 893–904 (2007).
Saederup, N. et al. Selective chemokine receptor usage by central nervous system myeloid cells in Ccr2-red fluorescent protein knock-in mice. PLoS ONE 5, e13693 (2010).
Umekawa, T., Osman, A. M., Han, W., Ikeda, T. & Blomgren, K. Resident microglia, rather than blood-derived macrophages, contribute to the earlier and more pronounced inflammatory reaction in the immature compared with the adult hippocampus after hypoxia-ischemia. Glia 63, 2220–2230 (2015).
Dal-Secco, D. et al. A dynamic spectrum of monocytes arising from the in situ reprogramming of Ccr2+ monocytes at a site of sterile injury. J. Exp. Med. 212, 447–456 (2015).
Chen, H. R. et al. Fate mapping via Ccr2-Creer mice reveals monocyte-to-microglia transition in development and neonatal stroke. Sci. Adv. 6, eabb2119 (2020).
Gliem, M. et al. Macrophages prevent hemorrhagic infarct transformation in murine stroke models. Ann. Neurol. 71, 743–752 (2012).
Pedragosa, J. et al. Ccr2 deficiency in monocytes impairs angiogenesis and functional recovery after ischemic stroke in mice. J. Cereb. Blood Flow. Metab. 40, S98–s116 (2020).
Pimentel-Coelho, P. M., Michaud, J. P. & Rivest, S. C-C Chemokine Receptor Type 2 (Ccr2) signaling protects neonatal male mice with hypoxic-ischemic hippocampal damage from developing spatial learning deficits. Behav. Brain Res 286, 146–151 (2015).
Hu, X. et al. Microglial and macrophage polarization—new prospects for brain repair. Nat. Rev. Neurol. 11, 56–64 (2015).
Chhor, V. et al. Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro. Brain Behav. Immun. 32, 70–85 (2013).
Hellstrom Erkenstam, N. et al. Temporal characterization of microglia/macrophage phenotypes in a mouse model of neonatal hypoxic-ischemic brain injury. Front. Cell Neurosci. 10, 286 (2016).
Kaminski, N. et al. Mesenchymal stromal cell-derived extracellular vesicles reduce neuroinflammation, promote neural cell proliferation and improve oligodendrocyte maturation in neonatal hypoxic-ischemic brain injury. Front. Cell Neurosci. 14, 601176 (2020).
Chhor, V. et al. Role of microglia in a mouse model of paediatric traumatic brain injury. Brain Behav. Immun. 63, 197–209 (2017).
Van Steenwinckel, J. et al. Decreased Microglial Wnt/beta-catenin signalling drives microglial pro-inflammatory activation in the developing brain. Brain 142, 3806–3833 (2019).
Villapol, S. et al. Early sex differences in the immune-inflammatory responses to neonatal ischemic stroke. Int. J. Mol. Sci. 20, 3809–3824 (2019).
Elmore, M. R. et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82, 380–397 (2014).
Hilla, A. M., Diekmann, H. & Fischer, D. Microglia are irrelevant for neuronal degeneration and axon regeneration after acute injury. J. Neurosci. 37, 6113–6124 (2017).
Otxoa-de-Amezaga, A. et al. Microglial cell loss after ischemic stroke favors brain neutrophil accumulation. Acta Neuropathol. 137, 321–341 (2019).
Tsuji, S. et al. Aggravated brain injury after neonatal hypoxic ischemia in microglia-depleted mice. J. Neuroinflammation 17, 111 (2020).
Taher, N. A. B. et al. Altered distributions and functions of natural killer T cells and gammadelta T cells in neonates with neonatal encephalopathy, in school-age children at follow-up, and in children with cerebral palsy. J. Neuroimmunol. 356, 577597 (2021).
Gan, Y. et al. Ischemic neurons recruit natural killer cells that accelerate brain infarction. Proc. Natl Acad. Sci. USA 111, 2704–2709 (2014).
Li, M. et al. Astrocyte-derived interleukin-15 exacerbates ischemic brain injury via propagation of cellular immunity. Proc. Natl Acad. Sci. USA 114, E396–E405 (2017).
He, H. et al. Nk cells promote neutrophil recruitment in the brain during sepsis-induced neuroinflammation. Sci. Rep. 6, 27711 (2016).
Albertsson, A. M. et al. Gammadelta T cells contribute to injury in the developing brain. Am. J. Pathol. 188, 757–767 (2018).
Gelderblom, M., Arunachalam, P. & Magnus, T. Gammadelta T cells as early sensors of tissue damage and mediators of secondary neurodegeneration. Front. Cell Neurosci. 8, 368 (2014).
Benakis, C. et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat. Med. 22, 516–523 (2016).
Goyal, M. S., Venkatesh, S., Milbrandt, J., Gordon, J. I. & Raichle, M. E. Feeding the brain and nurturing the mind: linking nutrition and the gut microbiota to brain development. Proc. Natl Acad. Sci. USA 112, 14105–14112 (2015).
Cahenzli, J., Koller, Y., Wyss, M., Geuking, M. B. & McCoy, K. D. Intestinal microbial diversity during early-life colonization shapes long-term IgE levels. Cell Host Microbe 14, 559–570 (2013).
Wesemann, D. R. et al. Microbial colonization influences early B-lineage development in the gut lamina propria. Nature 501, 112–115 (2013).
Fulde, M. et al. Neonatal selection by Toll-like receptor 5 influences long-term gut microbiota composition. Nature 560, 489–493 (2018).
Zhang, X. et al. Gammadeltat cells but not alphabetat cells contribute to sepsis-induced white matter injury and motor abnormalities in mice. J. Neuroinflammation 14, 255 (2017).
Albertsson, A. M. et al. The immune response after hypoxia-ischemia in a mouse model of preterm brain injury. J. Neuroinflammation 11, 153 (2014).
Benjelloun, N., Renolleau, S., Represa, A., Ben-Ari, Y. & Charriaut-Marlangue, C. Inflammatory responses in the cerebral cortex after ischemia in the P7 neonatal rat. Stroke 30, 1916–1923 (1999). Discussion 1923-1914.
Nazmi, A. et al. Lymphocytes contribute to the pathophysiology of neonatal brain injury. Front. Neurol. 9, 159 (2018).
Winerdal, M. et al. Adenosine A1 receptors contribute to immune regulation after neonatal hypoxic ischemic brain injury. Purinergic Signal. 12, 89–101 (2016).
Ogawa, Y., Tanaka, E., Sato, Y. & Tsuji, M. Brain damage caused by neonatal hypoxia-ischemia and the effects of hypothermia in severe combined immunodeficient (Scid) Mice. Exp. Neurol. 337, 113577 (2021).
Kleinschnitz, C. et al. Early detrimental T-cell effects in experimental cerebral ischemia are neither related to adaptive immunity nor thrombus formation. Blood 115, 3835–3842 (2010).
Neumann, J. et al. Very-late-antigen-4 (Vla-4)-mediated brain invasion by neutrophils leads to interactions with microglia, increased ischemic injury and impaired behavior in experimental stroke. Acta Neuropathol. 129, 259–277 (2015).
Aktas, O., Kury, P., Kieseier, B. & Hartung, H. P. Fingolimod is a potential novel therapy for multiple sclerosis. Nat. Rev. Neurol. 6, 373–382 (2010).
Brinkmann, V. et al. Fingolimod (Fty720): discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug Discov. 9, 883–897 (2010).
Liesz, A. et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat. Med. 15, 192–199 (2009).
Jellema, R. K. et al. Mesenchymal stem cells induce T-cell tolerance and protect the preterm brain after global hypoxia-ischemia. PLoS ONE 8, e73031 (2013).
Hedtjarn, M., Mallard, C. & Hagberg, H. Inflammatory gene profiling in the developing mouse brain after hypoxia-ischemia. J. Cereb. Blood Flow. Metab. 24, 1333–1351 (2004).
Zhang, X. et al. Type I interferons protect neonates from acute inflammation through interleukin 10-producing B cells. J. Exp. Med. 204, 1107–1118 (2007).
Mallard, C., Ek, C. J. & Vexler, Z. S. The myth of the immature barrier systems in the developing brain: role in perinatal brain injury. J. Physiol. 596, 5655–5664 (2018).
Andersson, E. A., Mallard, C. & Ek, C. J. Circulating tight-junction proteins are potential biomarkers for blood-brain barrier function in a model of neonatal hypoxic/ischemic brain injury. Fluids Barriers CNS 18, 7 (2021).
Ek, C. J. et al. Brain barrier properties and cerebral blood flow in neonatal mice exposed to cerebral hypoxia-ischemia. J. Cereb. Blood Flow. Metab. 35, 818–827 (2015).
Mottahedin, A. et al. N-Acetylcysteine inhibits bacterial lipopeptide-mediated neutrophil transmigration through the choroid plexus in the developing brain. Acta Neuropathol. Commun. 8, 4 (2020).
Carloni, S. et al. Simvastatin reduces Caspase-3 activation and inflammatory markers induced by hypoxia-ischemia in the newborn rat. Neurobiol. Dis. 21, 119–126 (2006).
Herz, J. et al. Interaction between hypothermia and delayed mesenchymal stem cell therapy in neonatal hypoxic-ischemic brain injury. Brain Behav. Immun. 70, 118–130 (2018).
Wang, Y. et al. Changes of inflammatory cytokines and neurotrophins emphasized their roles in hypoxic-ischemic brain damage. Int. J. Neurosci. 123, 191–195 (2013).
Ransohoff, R. M., Kivisäkk, P. & Kidd, G. Three or more routes for leukocyte migration into the central nervous system. Nat. Rev. Immunol. 3, 569–581 (2003).
Llovera, G. et al. The choroid plexus is a key cerebral invasion route for T cells after stroke. Acta Neuropathol. 134, 851–868 (2017).
Wilson, E. H., Weninger, W. & Hunter, C. A. Trafficking of immune cells in the central nervous system. J. Clin. Invest. 120, 1368–1379 (2010).
Rayasam, A., Faustino, J., Lecuyer, M. & Vexler, Z. S. Neonatal stroke and Tlr1/2 ligand recruit myeloid cells through the choroid plexus in a Cx3cr1-Ccr2- and context-specific manner. J. Neurosci. 40, 3849–3861 (2020).
Cai, R. et al. Panoptic imaging of transparent mice reveals whole-body neuronal projections and skull–meninges connections. Nat. Neurosci. 22, 317–327 (2019).
Herisson, F. et al. Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nat. Neurosci. 21, 1209–1217 (2018).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).
Louveau, A. et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat. Neurosci. 21, 1380–1391 (2018).
Al Mamun, A., Yu, H., Romana, S. & Liu, F. Inflammatory responses are sex specific in chronic hypoxic-ischemic encephalopathy. Cell Transplant, https://doi.org/10.1177/0963689718766362 (2018).
Charriaut-Marlangue, C., Besson, V. C. & Baud, O. Sexually dimorphic outcomes after neonatal stroke and hypoxia-ischemia. Int. J. Mol. Sci. 19, 61–71 (2017).
Hill, C. A. & Fitch, R. H. Sex differences in mechanisms and outcome of neonatal hypoxia-ischemia in rodent models: implications for sex-specific neuroprotection in clinical neonatal practice. Neurol. Res. Int. 2012, 867531 (2012).
Mirza, M. A., Ritzel, R., Xu, Y., McCullough, L. D. & Liu, F. Sexually dimorphic outcomes and inflammatory responses in hypoxic-ischemic encephalopathy. J. Neuroinflammation 12, 32 (2015).
Murden, S. et al. Gender differences involved in the pathophysiology of the perinatal hypoxic-ischemic damage. Physiol. Res. 68, S207–S217 (2019).
Sheiner, E. et al. Gender does matter in perinatal medicine. Fetal Diagn. Ther. 19, 366–369 (2004).
Cheong, J. L. & Cowan, F. M. Neonatal arterial ischaemic stroke: obstetric issues. Semin. Fetal Neonatal Med. 14, 267–271 (2009).
Tsze, D. S. & Valente, J. H. Pediatric stroke: a review. Emerg. Med. Int. 2011, 734506 (2011).
Wood, T. R. et al. Variability and sex-dependence of hypothermic neuroprotection in a rat model of neonatal hypoxic-ischaemic brain injury: a single laboratory meta-analysis. Sci. Rep. 10, 10833 (2020).
Ardalan, M., Chumak, T., Vexler, Z. & Mallard, C. Sex-dependent effects of perinatal inflammation on the brain: implication for neuro-psychiatric disorders. Int. J. Mol. Sci. 20, 2270–2299 (2019).
Burnsed, J. C. et al. Hypoxia-ischemia and therapeutic hypothermia in the neonatal mouse brain—a longitudinal study. PLoS ONE 10, e0118889 (2015).
Charriaut-Marlangue, C. et al. Sex differences in the effects of Parp inhibition on microglial phenotypes following neonatal stroke. Brain Behav. Immun. 73, 375–389 (2018).
Chavez-Valdez, R. et al. Evidence for sexual dimorphism in the response to Tlr3 activation in the developing neonatal mouse brain: a pilot study. Front. Physiol. 10, 306 (2019).
Lenz, K. M. & McCarthy, M. M. A starring role for microglia in brain sex differences. Neuroscientist 21, 306–321 (2015).
Lenz, K. M., Nugent, B. M., Haliyur, R. & McCarthy, M. M. Microglia are essential to masculinization of brain and behavior. J. Neurosci. 33, 2761–2772 (2013).
Klein, S. L. & Flanagan, K. L. Sex differences in immune responses. Nat. Rev. Immunol. 16, 626–638 (2016).
Polanczyk, M. J. et al. Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J. Immunol. 173, 2227–2230 (2004).
Wang, C. et al. Oestrogen modulates experimental autoimmune encephalomyelitis and interleukin-17 production via programmed death 1. Immunology 126, 329–335 (2009).
Goodman, W. A. et al. Impaired estrogen signaling underlies regulatory T cell loss-of-function in the chronically inflamed intestine. Proc. Natl Acad. Sci. USA 117, 17166–17176 (2020).
Vasanthakumar, A. et al. Sex-specific adipose tissue imprinting of regulatory T cells. Nature 579, 581–585 (2020).
Carr, R. Neutrophil production and function in newborn infants. Br. J. Haematol. 110, 18–28 (2000).
Lawrence, S. M., Corriden, R. & Nizet, V. Age-appropriate functions and dysfunctions of the neonatal neutrophil. Front. Pediatr. 5, 23 (2017).
Melvan, J. N., Bagby, G. J., Welsh, D. A., Nelson, S. & Zhang, P. Neonatal sepsis and neutrophil insufficiencies. Int. Rev. Immunol. 29, 315–348 (2010).
Herz, J. et al. Role of neutrophils in exacerbation of brain injury after focal cerebral ischemia in hyperlipidemic mice. Stroke 46, 2916–2925 (2015).
Ferriero, D. M. Neonatal brain injury. N. Engl. J. Med. 351, 1985–1995 (2004).
Fleiss, B. & Gressens, P. Tertiary mechanisms of brain damage: a new hope for treatment of cerebral palsy? Lancet Neurol. 11, 556–566 (2012).
Cuartero, M. I. et al. N2 neutrophils, novel players in brain inflammation after stroke: modulation by the PPARgamma agonist Rosiglitazone. Stroke 44, 3498–3508 (2013).
Marwick, J. A. et al. Neutrophils induce macrophage anti-inflammatory reprogramming by suppressing Nf-KappaB activation. Cell Death Dis. 9, 665 (2018).
Ortega-Gomez, A., Perretti, M. & Soehnlein, O. Resolution of inflammation: an integrated view. EMBO Mol. Med. 5, 661–674 (2013).
Peiseler, M. & Kubes, P. More friend than foe: the emerging role of neutrophils in tissue repair. J. Clin. Invest. 129, 2629–2639 (2019).
Sas, A. R. et al. A new neutrophil subset promotes cns neuron survival and axon regeneration. Nat. Immunol. 21, 1496–1505 (2020).
Kraft, P. et al. Fty720 ameliorates acute ischemic stroke in mice by reducing thrombo-inflammation but not by direct neuroprotection. Stroke 44, 3202–3210 (2013).
Hayakawa, S., Ohno, N., Okada, S. & Kobayashi, M. Significant augmentation of regulatory T cell numbers occurs during the early neonatal period. Clin. Exp. Immunol. 190, 268–279 (2017).
Prabhu, S. B. et al. Comparison of human neonatal and adult blood leukocyte subset composition phenotypes. PLoS One 11, e0162242 (2016).
Rueda, C. M. et al. Neonatal regulatory T cells have reduced capacity to suppress dendritic cell function. Eur. J. Immunol. 45, 2582–2592 (2015).
Craig, A. et al. Quantitative analysis of perinatal rodent oligodendrocyte lineage progression and its correlation with human. Exp. Neurol. 181, 231–240 (2003).
Gerstner, B. et al. Hyperoxia causes maturation-dependent cell death in the developing white matter. J. Neurosci. 28, 1236–1245 (2008).
White, J. R. et al. Evaluation of hematologic variables in newborn C57/Bl6 mice up to day 35. Vet. Clin. Pathol. 45, 87–95 (2016).
Correa-Rocha, R. et al. Preterm neonates show marked leukopenia and lymphopenia that are associated with increased regulatory T-cell values and diminished Il-7. Pediatr. Res. 71, 590–597 (2012).
Pagel, J. et al. Increased regulatory T cells precede the development of bronchopulmonary dysplasia in preterm infants. Front Immunol. 11, 565257 (2020).
Yellowhair, T. R. et al. Cxcr2 blockade mitigates neural cell injury following preclinical chorioamnionitis. Front. Physiol. 10, 324 (2019).
Jenkins, D. D. et al. Altered circulating leukocytes and their chemokines in a clinical trial of therapeutic hypothermia for neonatal hypoxic ischemic encephalopathy. Pediatr. Crit. Care Med. 14, 786–795 (2013).
Chu, H. X. et al. Immune cell infiltration in malignant middle cerebral artery infarction: comparison with transient cerebral ischemia. J. Cereb. Blood Flow. Metab. 34, 450–459 (2014).
Liesz, A. et al. Fty720 reduces post-ischemic brain lymphocyte influx but does not improve outcome in permanent murine cerebral ischemia. PLoS ONE 6, e21312 (2011).
Zhou, W. et al. Postischemic brain infiltration of leukocyte subpopulations differs among murine permanent and transient focal cerebral ischemia models. Brain Pathol. 23, 34–44 (2013).
Le Blanc, K. & Mougiakakos, D. Multipotent mesenchymal stromal cells and the innate immune system. Nat. Rev. Immunol. 12, 383–396 (2012).
Uccelli, A., Moretta, L. & Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 8, 726–736 (2008).
Donega, V. et al. Intranasally administered mesenchymal stem cells promote a regenerative niche for repair of neonatal ischemic brain injury. Exp. Neurol. 261, 53–64 (2014).
Jellema, R. K. et al. Multipotent adult progenitor cells for hypoxic-ischemic injury in the preterm brain. J. Neuroinflammation 12, 241 (2015).
Nair, S. et al. Neuroprotection offered by mesenchymal stem cells in perinatal brain injury: role of mitochondria, inflammation, and reactive oxygen species. J. Neurochem. 158, 59–73 (2020).
Ophelders, D. R. et al. Mesenchymal stromal cell-derived extracellular vesicles protect the fetal brain after hypoxia-ischemia. Stem Cells Transl. Med. 5, 754–763 (2016).
Passera, S. et al. Therapeutic potential of stem cells for preterm infant brain damage: can we move from the heterogeneity of preclinical and clinical studies to established therapeutics? Biochem. Pharmacol. 186, 114461 (2021).
Robertson, N. J. et al. Human umbilical cord mesenchymal stromal cells as an adjunct therapy with therapeutic hypothermia in a piglet model of perinatal asphyxia. Cytotherapy 23, 521–535 (2021).
Sisa, C. et al. Mesenchymal stromal cell derived extracellular vesicles reduce hypoxia-ischaemia induced perinatal brain injury. Front. Physiol. 10, 282 (2019).
van Velthoven, C. T., Kavelaars, A., van Bel, F. & Heijnen, C. J. Mesenchymal stem cell treatment after neonatal hypoxic-ischemic brain injury improves behavioral outcome and induces neuronal and oligodendrocyte regeneration. Brain Behav. Immun. 24, 387–393 (2010).
Farfan, N. et al. Intranasal administration of mesenchymal stem cell secretome reduces hippocampal oxidative stress, neuroinflammation and cell death, improving the behavioral outcome following perinatal asphyxia. Int. J. Mol. Sci. 21, 7800–7827 (2020).
McDonald, C. A. et al. Intranasal delivery of mesenchymal stromal cells protects against neonatal hypoxic(-)ischemic brain injury. Int. J. Mol. Sci. 20, 2449–2464 (2019).
Zhuang, X. et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 19, 1769–1779 (2011).
Rayasam, A., Fukuzaki, Y. & Vexler, Z. S. Microglia-leucocyte axis in cerebral ischaemia and inflammation in the developing brain. Acta Physiol. (Oxf), 233, e13674 (2021).
Sorokin, Y. et al. Umbilical cord serum interleukin-6, c-reactive protein, and myeloperoxidase concentrations at birth and association with neonatal morbidities and long-term neurodevelopmental outcomes. Am. J. Perinatol. 31, 717–726 (2014).
Ruhfus, M. et al. Association of routinely measured proinflammatory biomarkers with abnormal MRI findings in asphyxiated neonates undergoing therapeutic hypothermia. Front. Pediatr. 9, 624652 (2021).
Doeppner, T. R. et al. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl. Med. 4, 1131–1143 (2015).
Pasciuto, E. et al. Microglia require Cd4 T cells to complete the fetal-to-adult transition. Cell 182, 625–640 e624 (2020).
Tanabe, S. & Yamashita, T. B-1a lymphocytes promote oligodendrogenesis during brain development. Nat. Neurosci. 21, 506–516 (2018).
Bendix, I., Hadamitzky, M., Herz, J. & Felderhoff-Muser, U. Adverse neuropsychiatric development following perinatal brain injury: from a preclinical perspective. Pediatr. Res 85, 198–215 (2019).
Chen, H. R. et al. Monocytic infiltrates contribute to autistic-like behaviors in a two-hit model of neurodevelopmental defects. J. Neurosci. 40, 9386–9400 (2020).
Funding
Else-Kröner-Fresenius-Stiftung (2018_A113), European Union (Horizon 2020 Framework Program of the European Union, grant agreement no. 874721/ PREMSTEM). Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
J.H., I.B. and U.F.-M. conceptualized the manuscript. J.H. and I.B. performed literature research. J.H. drafted the manuscript. J.H., I.B. and U.F.-M. revised the manuscript draft.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Herz, J., Bendix, I. & Felderhoff-Müser, U. Peripheral immune cells and perinatal brain injury: a double-edged sword?. Pediatr Res 91, 392–403 (2022). https://doi.org/10.1038/s41390-021-01818-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01818-7