Abstract
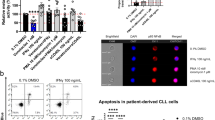
Resistance, to therapeutic antibodies used to treat chronic lymphocytic leukemia (CLL) patients is common. Monocyte-derived macrophages (MDMs) are a major effector of antitumour responses to therapeutic antibodies and we have previously reported that resistance to therapeutic antibodies, by MDMs, increases as CLL disease progresses. In this study, we examine the effect of a Class IIa-selective HDAC inhibitor (TMP195) on the phagocytic response to opsonised tumor cells or non-opsonised targets by MDMs derived from CLL patients. We report that TMP195 enhances phagocytic responses to antibody-opsonised CLL cells and E. coli within 30 min of treatment. The enhanced response is phenocopied by knockdown of the Class IIa HDAC, HDAC7, or by low concentrations of the pan-HDAC inhibitor, vorinostat. HDAC7 knockdown and inhibition induces hyperacetylation and hyperphosphorylation of Bruton’s tyrosine kinase (BTK). Moreover, BTK inhibitors abrogated the enhanced response to HDAC7 inhibition. Our data show that HDAC7 is an actionable driver of resistance to therapeutic antibodies by MDMs derived from CLL patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
08 February 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41388-020-01619-y
22 December 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41388-020-01619-y
References
Bosch F, Dalla-Favera R. Chronic lymphocytic leukaemia: from genetics to treatment. Nat Rev Clin Oncol. 2019;16:684–701.
Burgess M, Mapp S, Mazzieri R, Cheung C, Chambers L, Mattarollo SR, et al. Increased FcgammaRIIB dominance contributes to the emergence of resistance to therapeutic antibodies in chronic lymphocytic leukaemia patients. Oncogene. 2017;36:2366–76.
Chen YCE, Mapp S, Blumenthal A, Burgess ML, Mazzieri R, Mattarollo SR, et al. The duality of macrophage function in chronic lymphocytic leukaemia. Biochim Biophys Acta Rev Cancer. 2017;1868:176–82.
Hanna BS, McClanahan F, Yazdanparast H, Zaborsky N, Kalter V, Rossner PM, et al. Depletion of CLL-associated patrolling monocytes and macrophages controls disease development and repairs immune dysfunction in vivo. Leukemia. 2016;30:570–9.
Galletti G, Scielzo C, Barbaglio F, Rodriguez TV, Riba M, Lazarevic D, et al. Targeting macrophages sensitizes chronic lymphocytic leukemia to apoptosis and inhibits disease progression. Cell Rep. 2016;14:1748–60.
Salvagno C, Ciampricotti M, Tuit S, Hau CS, van Weverwijk A, Coffelt SB, et al. Therapeutic targeting of macrophages enhances chemotherapy efficacy by unleashing type I interferon response. Nat Cell Biol. 2019;21:511–21.
Guerriero JL, Sotayo A, Ponichtera HE, Castrillon JA, Pourzia AL, Schad S, et al. Class IIa HDAC inhibition reduces breast tumours and metastases through anti-tumour macrophages. Nature. 2017;543:428–32.
Barkal AA, Brewer RE, Markovic M, Kowarsky M, Barkal SA, Zaro BW, et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature. 2019;572:392–6.
Enya Chen YC, Burgess M, Mapp S, Mollee P, Gill D, Blumenthal A, et al. PI3K-p110delta contributes to antibody responses by macrophages in chronic lymphocytic leukemia. Leukemia. 2020;35:451–61.
Church AK, VanDerMeid KR, Baig NA, Baran AM, Witzig TE, Nowakowski GS, et al. Anti-CD20 monoclonal antibody-dependent phagocytosis of chronic lymphocytic leukaemia cells by autologous macrophages. Clin Exp Immunol. 2016;183:90–101.
VanDerMeid KR, Elliott MR, Baran AM, Barr PM, Chu CC, Zent CS. Cellular cytotoxicity of next-generation CD20 monoclonal antibodies. Cancer Immunol Res. 2018;6:1150–60.
Gul N, Babes L, Siegmund K, Korthouwer R, Bogels M, Braster R, et al. Macrophages eliminate circulating tumor cells after monoclonal antibody therapy. J Clin Investig. 2014;124:812–23.
Uribe-Querol E, Rosales C. Control of phagocytosis by microbial pathogens. Front Immunol. 2017;8:1368.
Veillette A, Chen J. SIRPalpha-CD47 immune checkpoint blockade in anticancer therapy. Trends Immunol. 2018;39:173–84.
Weiskopf K. Cancer immunotherapy targeting the CD47/SIRPalpha axis. Eur J Cancer. 2017;76:100–9.
Burgess M, Gill D, Singhania R, Cheung C, Chambers L, Renyolds BA, et al. CD62L as a therapeutic target in chronic lymphocytic leukemia. Clin Cancer Res. 2013;19:5675–85.
Roghanian A, Teige I, Martensson L, Cox KL, Kovacek M, Ljungars A, et al. Antagonistic human FcgammaRIIB (CD32B) antibodies have anti-tumor activity and overcome resistance to antibody therapy in vivo. Cancer Cell. 2015;27:473–88.
Murray PJ. Macrophage polarization. Annu Rev Physiol. 2017;79:541–66.
Ginhoux F, Schultze JL, Murray PJ, Ochando J, Biswas SK. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat Immunol. 2016;17:34–40.
Kimbrough D, Wang SH, Wright LH, Mani SK, Kasiganesan H, LaRue AC, et al. HDAC inhibition helps post-MI healing by modulating macrophage polarization. J Mol Cell Cardiol. 2018;119:51–63.
Qi X, Wang P. Class IIa HDACs inhibitor TMP269 promotes M1 polarization of macrophages after spinal cord injury. J Cell Biochem. 2018;119:3081–90.
Bobrowicz M, Dwojak M, Pyrzynska B, Stachura J, Muchowicz A, Berthel E, et al. HDAC6 inhibition upregulates CD20 levels and increases the efficacy of anti-CD20 monoclonal antibodies. Blood. 2017;130:1628–38.
Lobera M, Madauss KP, Pohlhaus DT, Wright QG, Trocha M, Schmidt DR, et al. Selective class IIa histone deacetylase inhibition via a nonchelating zinc-binding group. Nat Chem Biol. 2013;9:319–25.
Boissard F, Fournie JJ, Laurent C, Poupot M, Ysebaert L. Nurse like cells: chronic lymphocytic leukemia associated macrophages. Leuk Lymphoma. 2015;56:1570–2.
Ribes S, Ebert S, Regen T, Agarwal A, Tauber SC, Czesnik D, et al. Toll-like receptor stimulation enhances phagocytosis and intracellular killing of nonencapsulated and encapsulated Streptococcus pneumoniae by murine microglia. Infect Immun. 2010;78:865–71.
Khan N, Jeffers M, Kumar S, Hackett C, Boldog F, Khramtsov N, et al. Determination of the class and isoform selectivity of small-molecule histone deacetylase inhibitors. Biochemical J. 2007;409:581–9.
Marek L, Hamacher A, Hansen FK, Kuna K, Gohlke H, Kassack MU, et al. Histone deacetylase (HDAC) inhibitors with a novel connecting unit linker region reveal a selectivity profile for HDAC4 and HDAC5 with improved activity against chemoresistant cancer cells. J Medicinal Chem. 2013;56:427–36.
Liu Z, Mai A, Sun J. Lysine acetylation regulates Bruton’s tyrosine kinase in B cell activation. J Immunol. 2010;184:244–54.
Ackerman ME, Moldt B, Wyatt RT, Dugast A-S, McAndrew E, Tsoukas S, et al. A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. J Immunol Methods. 2011;366:8–19.
Van Damme M, Crompot E, Meuleman N, Mineur P, Dessars B, El Housni H, et al. Global histone deacetylase enzymatic activity is an independent prognostic marker associated with a shorter overall survival in chronic lymphocytic leukemia patients. Epigenetics. 2014;9:1374–81.
Jung JW, Veitch M, Bridge JA, Overgaard NH, Cruz JL, Linedale R, et al. Clinically-relevant rapamycin treatment regimens enhance CD8(+) effector memory T cell function in the skin and allow their infiltration into cutaneous squamous cell carcinoma. Oncoimmunology. 2018;7:e1479627.
Ramsay HM, Fryer AA, Hawley CM, Smith AG, Harden PN. Non-melanoma skin cancer risk in the Queensland renal transplant population. Br J Dermatol. 2002;147:950–6.
Lindelof B, Sigurgeirsson B, Gabel H, Stern RS. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol. 2000;143:513–9.
Varughese T, Taur Y, Cohen N, Palomba ML, Seo SK, Hohl TM, et al. Serious infections in patients receiving ibrutinib for treatment of lymphoid cancer. Clin Infect Dis Off Publ Infect Dis Soc Am. 2018;67:687–92.
Rogers KA, Mousa L, Zhao Q, Bhat SA, Byrd JC, E Boghdadly Z, et al. Incidence of opportunistic infections during ibrutinib treatment for B-cell malignancies. Leukemia. 2019;33:2527–30.
Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–56.
Burgess M, Cheung C, Chambers L, Ravindranath K, Minhas G, Knop L, et al. CCL2 and CXCL2 enhance survival of primary chronic lymphocytic leukemia cells in vitro. Leuk Lymphoma. 2012;53:1988–98.
Acknowledgements
The authors acknowledge the generosity of the patients who donated their time and blood for this study. This work was supported by a generous donation from Jeff and Fran Maclean and a research support package to DG from Queensland Health, Princess Alexandra Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Burgess, M., Chen, Y.C.E., Mapp, S. et al. HDAC7 is an actionable driver of therapeutic antibody resistance by macrophages from CLL patients. Oncogene 39, 5756–5767 (2020). https://doi.org/10.1038/s41388-020-01394-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-020-01394-w
This article is cited by
-
mPPTMP195 nanoparticles enhance fracture recovery through HDAC4 nuclear translocation inhibition
Journal of Nanobiotechnology (2024)
-
Clinical relevance of tumour-associated macrophages
Nature Reviews Clinical Oncology (2022)