Abstract
Background
Excessive visceral obesity in recipients of living donor liver transplantation (LDLT) is associated with mortality, and a recent study reported the correlation between visceral adiposity of male LDLT recipients and hepatocellular carcinoma (HCC) recurrence. However, there is no study on the relationship between the donor’s visceral adiposity and surgical outcomes in LDLT recipients. We investigated the association of the visceral-to-subcutaneous fat area ratio (VSR) in donors and recipients with HCC recurrence and mortality in LDLT.
Methods
We analyzed 1386 sets of donors and recipients who underwent LDLT between January 2008 and January 2018. The maximal chi-square method was used to determine the optimal cutoff values for VSR for predicting overall HCC recurrence and mortality. Cox regression analyses were performed to evaluate the association of donor VSR and recipient VSR with overall HCC recurrence and mortality in recipients.
Results
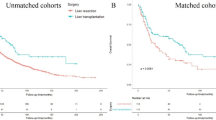
The cutoff values of VSR was determined as 0.73 in males and 0.31 in females. High donor VSR was significantly associated with overall HCC recurrence (adjusted hazard ratio [HR]: 1.43, 95% confidence interval [CI]: 1.06–1.93, p = 0.019) and mortality (HR: 1.35, 95% CI: 1.03–1.76, p = 0.030). High recipient VSR was significantly associated with overall HCC recurrence (HR: 1.40, 95% CI: 1.04–1.88, p = 0.027) and mortality (HR: 1.50, 95% CI: 1.14–1.96, p = 0.003).
Conclusions
Both recipient VSR and donor VSR were significant risk factors for HCC recurrence and mortality in LDLT recipients. Preoperative donor VSR and recipient VSR may be strong predictors of the surgical outcomes of LDLT recipients with HCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The dataset used and/or analyzed during the current study is available from the corresponding author upon reasonable request.
References
Sapisochin G, Bruix J. Liver transplantation for hepatocellular carcinoma: outcomes and novel surgical approaches. Nat Rev Gastroenterol Hepatol. 2017;14:203–17.
de’Angelis N, Landi F, Carra MC, Azoulay D. Managements of recurrent hepatocellular carcinoma after liver transplantation: A systematic review. World J Gastroenterol. 2015;21:11185–98.
Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, et al. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl. 2004;10:534–40.
Sapisochin G, Goldaracena N, Astete S, Laurence JM, Davidson D, Rafael E, et al. Benefit of Treating Hepatocellular Carcinoma Recurrence after Liver Transplantation and Analysis of Prognostic Factors for Survival in a Large Euro-American Series. Ann Surg Oncol. 2015;22:2286–94.
Koh PS, Chan SC. Adult-to-adult living donor liver transplantation: Operative techniques to optimize the recipient’s outcome. J Nat Sci Biol Med. 2017;8:4–10.
Brown RS Jr., Russo MW, Lai M, Shiffman ML, Richardson MC, Everhart JE, et al. A survey of liver transplantation from living adult donors in the United States. N Engl J Med. 2003;348:818–25.
Fahrner R, Dondorf F, Ardelt M, Dittmar Y, Settmacher U, Rauchfuß F. Liver transplantation for hepatocellular carcinoma - factors influencing outcome and disease-free survival. World J Gastroenterol. 2015;21:12071–82.
Nagai S, Yoshida A, Facciuto M, Moonka D, Abouljoud MS, Schwartz ME, et al. Ischemia time impacts recurrence of hepatocellular carcinoma after liver transplantation. Hepatology. 2015;61:895–904.
Agopian VG, Harlander-Locke M, Zarrinpar A, Kaldas FM, Farmer DG, Yersiz H, et al. A novel prognostic nomogram accurately predicts hepatocellular carcinoma recurrence after liver transplantation: analysis of 865 consecutive liver transplant recipients. J Am Coll Surg. 2015;220:416–27.
Shuster A, Patlas M, Pinthus JH, Mourtzakis M. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiology. 2012;85:1–10.
Unamuno X, Gómez-Ambrosi J, Rodríguez A, Becerril S, Frühbeck G, Catalán V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur J Clin Invest. 2018;48:e12997.
Kang HW, Kim D, Kim HJ, Kim CH, Kim YS, Park MJ, et al. Visceral obesity and insulin resistance as risk factors for colorectal adenoma: a cross-sectional, case-control study. Am J Gastroenterol. 2010;105:178–87.
Schapira DV, Clark RA, Wolff PA, Jarrett AR, Kumar NB, Aziz NM. Visceral obesity and breast cancer risk. Cancer. 1994;74:632–9.
Montano-Loza AJ, Mazurak VC, Ebadi M, Meza-Junco J, Sawyer MB, Baracos VE, et al. Visceral adiposity increases risk for hepatocellular carcinoma in male patients with cirrhosis and recurrence after liver transplant. Hepatology. 2018;67:914–23.
Ha Y, Kim D, Han S, Chon YE, Lee YB, Kim MN, et al. Sarcopenia Predicts Prognosis in Patients with Newly Diagnosed Hepatocellular Carcinoma, Independent of Tumor Stage and Liver Function. Cancer Res Treat. 2018;50:843–51.
Lee SG. A complete treatment of adult living donor liver transplantation: a review of surgical technique and current challenges to expand indication of patients. Am J Transplant. 2015;15:17–38.
Park HJ, Shin Y, Park J, Kim H, Lee IS, Seo DW, et al. Development and Validation of a Deep Learning System for Segmentation of Abdominal Muscle and Fat on Computed Tomography. Korean J Radiol. 2020;21:88–100.
Ouh Y-T, Lee K-M, Ahn KH, Hong S-C, Oh M-J, Kim H-J, et al. Predicting peripartum blood transfusion: focusing on pre-pregnancy characteristics. BMC Pregnancy Childbirth. 2019;19:477.
Park J, Gil JR, Shin Y, Won SE, Huh J, You MW, et al. Reliable and robust method for abdominal muscle mass quantification using CT/MRI: An explorative study in healthy subjects. PLoS One. 2019;14:e0222042.
Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006.
Montano-Loza AJ, Duarte-Rojo A, Meza-Junco J, Baracos VE, Sawyer MB, Pang JX, et al. Inclusion of Sarcopenia Within MELD (MELD-Sarcopenia) and the Prediction of Mortality in Patients With Cirrhosis. Clin Transl Gastroenterol. 2015;6:e102.
Pham HP, Shaz BH. Update on massive transfusion. Br J Anaesth. 2013;111:i71–82.
Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Shirai H, Yao S, et al. Preoperative Visceral Adiposity and Muscularity Predict Poor Outcomes after Hepatectomy for Hepatocellular Carcinoma. Liver Cancer. 2019;8:92–109.
Kubota T, Hata K, Sozu T, Ueda Y, Hirao H, Okamura Y, et al. Impact of Donor Age on Recipient Survival in Adult-to-Adult Living-donor Liver Transplantation. Ann Surg. 2018;267:1126–33.
Abt PL, Mange KC, Olthoff KM, Markmann JF, Reddy KR, Shaked A. Allograft survival following adult-to-adult living donor liver transplantation. Am J Transplant. 2004;4:1302–7.
McCormack L, Dutkowski P, El-Badry AM, Clavien PA. Liver transplantation using fatty livers: always feasible? J Hepatol. 2011;54:1055–62.
Takagi K, de Wilde RF, Polak WG. The effect of donor body mass index on graft function in liver transplantation: A systematic review. Transplant Rev (Orlando). 2020;34:100571.
Berzigotti A, Saran U, Dufour JF. Physical activity and liver diseases. Hepatology. 2016;63:1026–40.
Pantanetti P, Garrapa GG, Mantero F, Boscaro M, Faloia E, Venarucci D. Adipose tissue as an endocrine organ? A review of recent data related to cardiovascular complications of endocrine dysfunctions. Clin Exp Hypertens. 2004;26:387–98.
Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–83.
Lutz CT, Quinn LS. Sarcopenia, obesity, and natural killer cell immune senescence in aging: altered cytokine levels as a common mechanism. Aging (Albany NY). 2012;4:535–46.
Milner JJ, Beck MA. The impact of obesity on the immune response to infection. Proc Nutr Soc. 2012;71:298–306.
Briceño J, Ciria R. Early graft dysfunction after liver transplantation. Transplant Proc. 2010;42:631–3.
Fain JN, Bahouth SW, Madan AK. TNFalpha release by the nonfat cells of human adipose tissue. Int J Obes Relat Metab Disord. 2004;28:616–22.
Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam Horm. 2006;74:443–77.
Rytka JM, Wueest S, Schoenle EJ, Konrad D. The portal theory supported by venous drainage-selective fat transplantation. Diabetes. 2011;60:56–63.
Schwenzer NF, Machann J, Schraml C, Springer F, Ludescher B, Stefan N, et al. Quantitative analysis of adipose tissue in single transverse slices for estimation of volumes of relevant fat tissue compartments: a study in a large cohort of subjects at risk for type 2 diabetes by MRI with comparison to anthropometric data. Invest Radiol. 2010;45:788–94.
Després JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126:1301–13.
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), which is funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HR20C0026), and was supported by Institute of Information & communications Technology Planning & Evaluation (IITP) grant funded by the Korea government (MSIT, Project No. 2021-0-00393). This study was also supported by a grant (2022IP0053) from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea).
Author information
Authors and Affiliations
Contributions
JHS and JGS conceived and designed the study; JHS, KYK, YSK, HMK, YJM, IGJ, SHK, and JGS were involved in data acquisition; JHS, HMK, IGJ, and GSH were involved in the analysis and/or interpretation of data; JHS drafted the manuscript; JGS revised the manuscript critically for important intellectual content. All authors gave approval for the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
The institutional review board of Asan Medical Center (Protocol No. 2021-0526) approved this study. The need for informed consent from individual patients was waived owing to the retrospective nature of the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41366_2023_1367_MOESM4_ESM.tif
Supplementary Figure 2. Kaplan–Meier curves adjusted by the donor and recipient sex for overall HCC recurrence and mortality according to donor VSR and recipient VSR
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sim, JH., Kim, KW., Ko, Y. et al. Association between visceral obesity and tumor recurrence in hepatocellular carcinoma recipients undergoing liver transplantation. Int J Obes 47, 1214–1223 (2023). https://doi.org/10.1038/s41366-023-01367-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-023-01367-5