Abstract
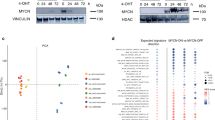
Retinoblastomas can arise from cone photoreceptor precursors in response to the loss of pRB function. Cone precursor-specific circuitry cooperates with pRB loss to initiate this process and subsequently contributes to the malignancy. Intrinsic high-level MDM2 expression is a key component of the cone precursor circuitry and is thought to inactivate p53-mediated tumor surveillance, which could otherwise be induced in response to pRB loss. However, the MDM2-related MDM4 has also been proposed to abrogate p53-mediated tumor surveillance in the absence of detectable MDM2 in retinoblastoma cells, bringing into question the importance of high-level MDM2 versus MDM4 expression. Here we report that high-level MDM2 but not MDM4 has a consistent critical role in retinoblastoma cell proliferation in vitro, as well as in orthotopic xenografts. Reduction of either MDM2 or MDM4 weakly induced p53, yet reduction of MDM2 but not MDM4 severely impaired proliferation and survival through a p53-independent mechanism. Specifically, MDM2 upregulated the mRNA expression and translation of another component of the cone circuitry, MYCN, in retinoblastoma cells. Moreover, MYCN was essential to retinoblastoma cell growth and tumor formation, and ectopic MYCN partially reversed the effects of MDM2 depletion, indicating that MYCN is an important MDM2 target. These findings indicate that high-level MDM2 expression is needed in order to perform a critical p53-independent function and may obviate the need for genomic alterations to the p53 pathway during retinoblastoma tumorigenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Dimaras H, Corson TW, Cobrinik D, White A, Zhao J, Munier FL et al. Retinoblastoma. Nat Rev Dis Primers 2015; 1: 15021.
Xu XL, Singh HP, Wang L, Qi DL, Poulos BK, Abramson DH et al. Rb suppresses human cone-precursor-derived retinoblastoma tumours. Nature 2014; 514: 385–388.
Xu XL, Fang Y, Lee TC, Forrest D, Gregory-Evans C, Almeida D et al. Retinoblastoma has properties of a cone precursor tumor and depends upon cone-specific MDM2 signaling. Cell 2009; 137: 1018–1031.
Kooi IE, Mol BM, Moll AC, van der Valk P, de Jong MC, de Graaf P et al. Loss of photoreceptorness and gain of genomic alterations in retinoblastoma reveal tumor progression. EBioMedicine 2015; 2: 660–670.
Cobrinik D . Retinoblastoma progression. EBioMedicine 2015; 2: 623–624.
Lee TC, Almeida D, Claros N, Abramson DH, Cobrinik D . Cell cycle-specific and cell type-specific expression of Rb in the developing human retina. Invest Ophthalmol Vis Sci 2006; 47: 5590–5598.
Wang H, Bauzon F, Ji P, Xu X, Sun D, Locker J et al. Skp2 is required for survival of aberrantly proliferating Rb1-deficient cells and for tumorigenesis in Rb1+/- mice. Nat Genet 2010; 42: 83–88.
Lowe SW, Cepero E, Evan G . Intrinsic tumour suppression. Nature 2004; 432: 307–315.
Cobrinik D . Pocket proteins and cell cycle control. Oncogene 2005; 24: 2796–2809.
Viatour P, Sage J . Newly identified aspects of tumor suppression by RB. Dis Model Mech 2011; 4: 581–585.
Chicas A, Wang X, Zhang C, McCurrach M, Zhao Z, Mert O et al. Dissecting the unique role of the retinoblastoma tumor suppressor during cellular senescence. Cancer Cell 2010; 17: 376–387.
Bates S, Phillips AC, Clark PA, Stott F, Peters G, Ludwig RL et al. p14ARF links the tumour suppressors RB and p53. Nature 1998; 395: 124–125.
Aslanian A, Iaquinta PJ, Verona R, Lees JA . Repression of the Arf tumor suppressor by E2F3 is required for normal cell cycle kinetics. Genes Dev 2004; 18: 1413–1422.
Komori H, Enomoto M, Nakamura M, Iwanaga R, Ohtani K . Distinct E2F-mediated transcriptional program regulates p14ARF gene expression. EMBO J 2005; 24: 3724–3736.
Lowe SW, Sherr CJ . Tumor suppression by Ink4a-Arf: progress and puzzles. Curr Opin Genet Dev 2003; 13: 77–83.
Sherr CJ, McCormick F . The RB and p53 pathways in cancer. Cancer Cell 2002; 2: 103–112.
Kato MV, Shimizu T, Ishizaki K, Kaneko A, Yandell DW, Toguchida J et al. Loss of heterozygosity on chromosome 17 and mutation of the p53 gene in retinoblastoma. Cancer Lett 1996; 106: 75–82.
Guo Y, Pajovic S, Gallie BL . Expression of p14ARF, MDM2, and MDM4 in human retinoblastoma. Biochem Biophys Res Commun 2008; 375: 1–5.
Harbour JW, Worley L, Ma D, Cohen M . Transducible peptide therapy for uveal melanoma and retinoblastoma. Arch Ophthalmol 2002; 120: 1341–1346.
Elison JR, Cobrinik D, Claros N, Abramson DH, Lee TC . Small molecule inhibition of HDM2 leads to p53-mediated cell death in retinoblastoma cells. Arch Ophthalmol 2006; 124: 1269–1275.
Laurie NA, Donovan SL, Shih CS, Zhang J, Mills N, Fuller C et al. Inactivation of the p53 pathway in retinoblastoma. Nature 2006; 444: 61–66.
McEvoy J, Flores-Otero J, Zhang J, Nemeth K, Brennan R, Bradley C et al. Coexpression of normally incompatible developmental pathways in retinoblastoma genesis. Cancer Cell 2011; 20: 260–275.
McEvoy J, Ulyanov A, Brennan R, Wu G, Pounds S, Zhang J et al. Analysis of MDM2 and MDM4 single nucleotide polymorphisms, mRNA splicing and protein expression in retinoblastoma. PLoS One 2012; 7: e42739.
Wade M, Wang YV, Wahl GM . The p53 orchestra: Mdm2 and Mdmx set the tone. Trends Cell Biol 2010; 20: 299–309.
Brennan RC, Federico S, Bradley C, Zhang J, Flores-Otero J, Wilson M et al. Targeting the p53 pathway in retinoblastoma with subconjunctival Nutlin-3a. Cancer Res 2011; 71: 4205–4213.
Thériault BL, Dimaras H, Gallie BL, Corson TW . The genomic landscape of retinoblastoma: a review. Clin Exp Ophthalmol 2014; 42: 33–52.
Efeyan A, Serrano M . p53: guardian of the genome and policeman of the oncogenes. Cell Cycle 2007; 6: 1006–1010.
Wang SP, Wang WL, Chang YL, Wu CT, Chao YC, Kao SH et al. p53 controls cancer cell invasion by inducing the MDM2-mediated degradation of Slug. Nat Cell Biol 2009; 11: 694–704.
Hu B, Gilkes DM, Farooqi B, Sebti SM, Chen J . MDMX overexpression prevents p53 activation by the MDM2 inhibitor Nutlin. J Biol Chem 2006; 281: 33030–33035.
Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV et al. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science 1999; 285: 1733–1737.
Sohn D, Graupner V, Neise D, Essmann F, Schulze-Osthoff K, Janicke RU . Pifithrin-alpha protects against DNA damage-induced apoptosis downstream of mitochondria independent of p53. Cell Death Differ 2009; 16: 869–878.
Liu DP, Song H, Xu Y . A common gain of function of p53 cancer mutants in inducing genetic instability. Oncogene 2010; 29: 949–956.
Terzian T, Suh YA, Iwakuma T, Post SM, Neumann M, Lang GA et al. The inherent instability of mutant p53 is alleviated by Mdm2 or p16INK4a loss. Genes Dev 2008; 22: 1337–1344.
Cobrinik D, Francis RO, Abramson DH, Lee TC . Rb induces a proliferative arrest and curtails Brn-2 expression in retinoblastoma cells. Mol Cancer 2006; 5: 72.
Evans L, Chen L, Milazzo G, Gherardi S, Perini G, Willmore E et al. SKP2 is a direct transcriptional target of MYCN and a potential therapeutic target in neuroblastoma. Cancer Lett 2015; 363: 37–45.
Dieterich DC, Link AJ, Graumann J, Tirrell DA, Schuman EM . Selective identification of newly synthesized proteins in mammalian cells using bioorthogonal noncanonical amino acid tagging (BONCAT). Proc Natl Acad Sci USA 2006; 103: 9482–9487.
Miles WO, Korenjak M, Griffiths LM, Dyer MA, Provero P, Dyson NJ . Post-transcriptional gene expression control by NANOS is up-regulated and functionally important in pRb-deficient cells. EMBO J 2014; 33: 2201–2215.
Efeyan A, Garcia-Cao I, Herranz D, Velasco-Miguel S, Serrano M . Tumour biology: policing of oncogene activity by p53. Nature 2006; 443: 159.
Christophorou MA, Ringshausen I, Finch AJ, Swigart LB, Evan GI . The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature 2006; 443: 214–217.
Robanus-Maandag E, Dekker M, van der Valk M, Carrozza ML, Jeanny JC, Dannenberg JH et al. p107 is a suppressor of retinoblastoma development in pRb-deficient mice. Genes Dev 1998; 12: 1599–1609.
Ajioka I, Martins RA, Bayazitov IT, Donovan S, Johnson DA, Frase S et al. Differentiated horizontal interneurons clonally expand to form metastatic retinoblastoma in mice. Cell 2007; 131: 378–390.
Gratias S, Schuler A, Hitpass LK, Stephan H, Rieder H, Schneider S et al. Genomic gains on chromosome 1q in retinoblastoma: consequences on gene expression and association with clinical manifestation. Int J Cancer 2005; 116: 555–563.
Wang YV, Wade M, Wong E, Li YC, Rodewald LW, Wahl GM . Quantitative analyses reveal the importance of regulated Hdmx degradation for p53 activation. Proc Natl Acad Sci USA 2007; 104: 12365–12370.
Li Q, Lozano G . Molecular pathways: targeting Mdm2 and Mdm4 in cancer therapy. Clin Cancer Res 2013; 19: 34–41.
To KH, Pajovic S, Gallie BL, Thériault BL . Regulation of p14ARF expression by miR-24: a potential mechanism compromising the p53 response during retinoblastoma development. BMC Cancer 2012; 12: 69.
Rushlow DE, Mol BM, Kennett JY, Yee S, Pajovic S, Thériault BL et al. Characterisation of retinoblastomas without RB1 mutations: genomic, gene expression, and clinical studies. Lancet Oncol 2013; 14: 327–334.
Huang M, Weiss WA . Neuroblastoma and MYCN. Cold Spring Harb Perspect Med 2013; 3: a014415.
Gu L, Zhang H, He J, Li J, Huang M, Zhou M . MDM2 regulates MYCN mRNA stabilization and translation in human neuroblastoma cells. Oncogene 2012; 31: 1342–1353.
Carr-Wilkinson J, O’Toole K, Wood KM, Challen CC, Baker AG, Board JR et al. High frequency of p53/MDM2/p14ARF pathway abnormalities in relapsed neuroblastoma. Clin Cancer Res 2010; 16: 1108–1118.
Cobrinik D, Ostrovnaya I, Hassimi M, Tickoo SK, Cheung IY, Cheung NK . Recurrent pre-existing and acquired DNA copy number alterations, including focal TERT gains, in neuroblastoma central nervous system metastases. Genes Chromosomes Cancer 2013; 52: 1150–1166.
Embade N, Fernandez-Ramos D, Varela-Rey M, Beraza N, Sini M, Gutierrez de Juan V et al. Murine double minute 2 regulates Hu antigen R stability in human liver and colon cancer through NEDDylation. Hepatology 2012; 55: 1237–1248.
Candeias MM, Malbert-Colas L, Powell DJ, Daskalogianni C, Maslon MM, Naski N et al. P53 mRNA controls p53 activity by managing Mdm2 functions. Nat Cell Biol 2008; 10: 1098–1105.
Barberi T, Bradbury M, Dincer Z, Panagiotakos G, Socci ND, Studer L . Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med 2007; 13: 642–648.
Acknowledgements
D–L Qi and D Cobrinik designed the study. D–L Qi conducted all the experiments. D–L Qi and D Cobrinik wrote the manuscript. We thank Xiaoliang Leon Xu for early passage retinoblastoma cell preparations, Narine Harutyunyan and Jennifer Aparicio for CHLAVC-RB43, Pat Reynolds and A Linn Murphree for CHLA-RB215 cells, and the Saban Research Institute Research Imaging Core for assistance. This study was supported by NIH grants 1R01CA137124 and P30CA014089, by a Saban Research Institute Research Career Development Fellowship to D–L Qi, by Research to Prevent Blindness (New York, New York), by the Larry & Celia Moh Foundation and by the charitable support of the Nautica Malibu Triathlon event produced by MESP, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Qi, DL., Cobrinik, D. MDM2 but not MDM4 promotes retinoblastoma cell proliferation through p53-independent regulation of MYCN translation. Oncogene 36, 1760–1769 (2017). https://doi.org/10.1038/onc.2016.350
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.350