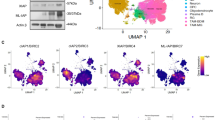
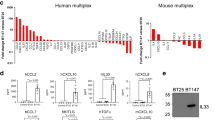
Abstract
Microglial cells in the brain tumor microenvironment are associated with enhanced glioma malignancy. They persist in an immunosuppressive M2 state at the peritumoral site and promote the growth of gliomas. Here, we investigated the underlying factors contributing to the abolished immune surveillance. We show that brain tumors escape pro-inflammatory M1 conversion of microglia via CD74 activation through the secretion of the cytokine macrophage migration inhibitory factor (MIF), which results in a M2 shift of microglial cells. Interruption of this glioma–microglial interaction through an antibody-neutralizing approach or small interfering RNA (siRNA)-mediated inhibition prolongs survival time in glioma-implanted mice by reinstating the microglial pro-inflammatory M1 function. We show that MIF-CD74 signaling inhibits interferon (IFN)-γ secretion in microglia through phosphorylation of microglial ERK1/2 (extracellular signal-regulated protein kinases 1 and 2). The inhibition of MIF signaling or its receptor CD74 promotes IFN-γ release and amplifies tumor death either through pharmacological inhibition or through siRNA-mediated knockdown. The reinstated IFN-γ secretion leads both to direct inhibition of glioma growth as well as inducing a M2 to M1 shift in glioma-associated microglia. Our data reveal that interference with the MIF signaling pathway represents a viable therapeutic option for the restoration of IFN-γ-driven immune surveillance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Dunn GP, Rinne ML, Wykosky J, Genovese G, Quayle SN, Dunn IF et al. Emerging insights into the molecular and cellular basis of glioblastoma. Genes Dev 2012; 26: 756–784.
Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT . Angiogenesis in brain tumours. Nat Rev Neurosci 2007; 8: 610–622.
Rao JS . Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer 2003; 3: 489–501.
Savaskan NE, Heckel A, Hahnen E, Engelhorn T, Doerfler A, Ganslandt O et al. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat Med 2008; 14: 629–632.
Saadoun S, Papadopoulos MC . Aquaporin-4 in brain and spinal cord oedema. Neuroscience 2010; 168: 1036–1046.
Lyons SA, Chung WJ, Weaver AK, Ogunrinu T, Sontheimer H . Autocrine glutamate signaling promotes glioma cell invasion. Cancer Res 2007; 67: 9463–9471.
Savaskan NE, Seufert S, Hauke J, Trankle C, Eyupoglu IY, Hahnen E . Dissection of mitogenic and neurodegenerative actions of cystine and glutamate in malignant gliomas. Oncogene 2011; 30: 43–53.
Engelhorn T, Savaskan NE, Schwarz MA, Kreutzer J, Meyer EP, Hahnen E et al. Cellular characterization of the peritumoral edema zone in malignant brain tumors. Cancer Sci 2009; 100: 1856–1862.
Zhai H, Heppner FL, Tsirka SE . Microglia/macrophages promote glioma progression. Glia 2011; 59: 472–485.
Eyupoglu IY, Bechmann I, Nitsch R . Modification of microglia function protects from lesion-induced neuronal alterations and promotes sprouting in the hippocampus. FASEB J 2003; 17: 1110–1111.
Ullrich O, Diestel A, Eyupoglu IY, Nitsch R . Regulation of microglial expression of integrins by poly(ADP-ribose) polymerase-1. Nat Cell Biol 2001; 3: 1035–1042.
Heneka MT . Macrophages derived from infiltrating monocytes mediate autoimmune myelin destruction. J Exp Med 2014; 211: 1500.
Komohara Y, Takemura K, Lei XF, Sakashita N, Harada M, Suzuki H et al. Delayed growth of EL4 lymphoma in SR-A-deficient mice is due to upregulation of nitric oxide and interferon-gamma production by tumor-associated macrophages. Cancer Sci 2009; 100: 2160–2166.
Komohara Y, Horlad H, Ohnishi K, Fujiwara Y, Bai B, Nakagawa T et al. Importance of direct macrophage-tumor cell interaction on progression of human glioma. Cancer Sci 2012; 103: 2165–2172.
Prosniak M, Harshyne LA, Andrews DW, Kenyon LC, Bedelbaeva K, Apanasovich TV et al. Glioma grade is associated with the accumulation and activity of cells bearing M2 monocyte markers. Clin Cancer Res 2013; 19: 3776–3786.
Ku MC, Wolf SA, Respondek D, Matyash V, Pohlmann A, Waiczies S et al. GDNF mediates glioblastoma-induced microglia attraction but not astrogliosis. Acta Neuropathol 2013; 125: 609–620.
Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF et al. CSF-1 R inhibition alters macrophage polarization and blocks glioma progression. Nat Med 2013; 19: 1264–1272.
Sielska M, Przanowski P, Wylot B, Gabrusiewicz K, Maleszewska M, Kijewska M et al. Distinct roles of CSF family cytokines in macrophage infiltration and activation in glioma progression and injury response. J Pathol 2013; 230: 310–321.
Wesolowska A, Kwiatkowska A, Slomnicki L, Dembinski M, Master A, Sliwa M et al. Microglia-derived TGF-beta as an important regulator of glioblastoma invasion—an inhibition of TGF-beta-dependent effects by shRNA against human TGF-beta type II receptor. Oncogene 2008; 27: 918–930.
Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med 2007; 13: 587–596.
Binsky I, Haran M, Starlets D, Gore Y, Lantner F, Harpaz N et al. IL-8 secreted in a macrophage migration-inhibitory factor- and CD74-dependent manner regulates B cell chronic lymphocytic leukemia survival. Proc Natl Acad Sci USA 2007; 104: 13408–13413.
Calandra T, Roger T . Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol 2003; 3: 791–800.
Leng L, Metz CN, Fang Y, Xu J, Donnelly S, Baugh J et al. MIF signal transduction initiated by binding to CD74. J Exp Med 2003; 197: 1467–1476.
Ren Y, Chan HM, Li Z, Lin C, Nicholls J, Chen CF et al. Upregulation of macrophage migration inhibitory factor contributes to induced N-Myc expression by the activation of ERK signaling pathway and increased expression of interleukin-8 and VEGF in neuroblastoma. Oncogene 2004; 23: 4146–4154.
Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia 2004; 6: 1–6.
Lubetsky JB, Dios A, Han J, Aljabari B, Ruzsicska B, Mitchell R et al. The tautomerase active site of macrophage migration inhibitory factor is a potential target for discovery of novel anti-inflammatory agents. J Biol Chem 2002; 277: 24976–24982.
Shi X, Leng L, Wang T, Wang W, Du X, Li J et al. CD44 is the signaling component of the macrophage migration inhibitory factor-CD74 receptor complex. Immunity 2006; 25: 595–606.
Fingerle-Rowson G, Kaleswarapu DR, Schlander C, Kabgani N, Brocks T, Reinart N et al. A tautomerase-null macrophage migration-inhibitory factor (MIF) gene knock-in mouse model reveals that protein interactions and not enzymatic activity mediate MIF-dependent growth regulation. Mol Cell Biol 2009; 29: 1922–1932.
Eyupoglu IY, Buchfelder M, Savaskan NE . Surgical resection of malignant gliomas-role in optimizing patient outcome. Nat Rev Neurol 2013; 9: 141–151.
Hanahan D, Weinberg RA . Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674.
Joyce JA, Pollard JW . Microenvironmental regulation of metastasis. Nat Rev Cancer 2009; 9: 239–252.
Baron N, Deuster O, Noelker C, Stuer C, Strik H, Schaller C et al. Role of macrophage migration inhibitory factor in primary glioblastoma multiforme cells. J Neurosci Res 2011; 89: 711–717.
Mittelbronn M, Platten M, Zeiner P, Dombrowski Y, Frank B, Zachskorn C et al. Macrophage migration inhibitory factor (MIF) expression in human malignant gliomas contributes to immune escape and tumour progression. Acta Neuropathol 2011; 122: 353–365.
Schwartz V, Lue H, Kraemer S, Korbiel J, Krohn R, Ohl K et al. A functional heteromeric MIF receptor formed by CD74 and CXCR4. FEBS Lett 2009; 583: 2749–2757.
Bryan KJ, Zhu X, Harris PL, Perry G, Castellani RJ, Smith MA et al. Expression of CD74 is increased in neurofibrillary tangles in Alzheimer's disease. Mol Neurodegener 2008; 3: 13.
Flynn G, Maru S, Loughlin J, Romero IA, Male D . Regulation of chemokine receptor expression in human microglia and astrocytes. J Neuroimmunol 2003; 136: 84–93.
Muller A, Brandenburg S, Turkowski K, Muller S, Vajkoczy P . Resident microglia, and not peripheral macrophages, are the main source of brain tumor mononuclear cells. Int J Cancer 2015; 137: 278–288.
Butrym A, Majewski M, Dzietczenia J, Kuliczkowski K, Mazur G . High CD74 expression correlates with ZAP70 expression in B cell chronic lymphocytic leukemia patients. Med Oncol 2013; 30: 560.
Kitange GJ, Carlson BL, Schroeder MA, Decker PA, Morlan BW, Wu W et al. Expression of CD74 in high grade gliomas: a potential role in temozolomide resistance. J Neurooncol 2010; 100: 177–186.
Nagata S, Jin YF, Yoshizato K, Tomoeda M, Song M, Iizuka N et al. CD74 is a novel prognostic factor for patients with pancreatic cancer receiving multimodal therapy. Ann Surg Oncol 2009; 16: 2531–2538.
Zeiner PS, Preusse C, Blank AE, Zachskorn C, Baumgarten P, Caspary L et al. MIF receptor CD74 is restricted to microglia/macrophages, associated with a M1-polarized immune milieu, and prolonged patient survival in gliomas. Brain Pathol 2015; 25: 491.
Gupta P, Goldenberg DM, Rossi EA, Cardillo TM, Byrd JC, Muthusamy N et al. Dual-targeting immunotherapy of lymphoma: potent cytotoxicity of anti-CD20/CD74 bispecific antibodies in mantle cell and other lymphomas. Blood 2012; 119: 3767–3778.
Becker-Herman S, Arie G, Medvedovsky H, Kerem A, Shachar I . CD74 is a member of the regulated intramembrane proteolysis-processed protein family. Mol Biol Cell 2005; 16: 5061–5069.
Wei J, Gabrusiewicz K, Heimberger A . The controversial role of microglia in malignant gliomas. Clin Dev Immunol 2013; 2013: 285246.
Calandra T, Bernhagen J, Mitchell RA, Bucala R . The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med 1994; 179: 1895–1902.
Yaddanapudi K, Rendon BE, Lamont G, Kim EJ, Al Rayyan N, Richie J et al. MIF is necessary for late-stage melanoma patient MDSC immune suppression and differentiation. Cancer Immunol Res 2015; 4: 101–112.
Kaufman JL, Niesvizky R, Stadtmauer EA, Chanan-Khan A, Siegel D, Horne H et al. Phase I, multicentre, dose-escalation trial of monotherapy with milatuzumab (humanized anti-CD74 monoclonal antibody) in relapsed or refractory multiple myeloma. Br J Haematol 2013; 163: 478–486.
Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol 2000; 20: 4106–4114.
Saura J, Tusell JM, Serratosa J . High-yield isolation of murine microglia by mild trypsinization. Glia 2003; 44: 183–189.
Smith AM, Gibbons HM, Lill C, Faull RL, Dragunow M . Isolation and culture of adult human microglia within mixed glial cultures for functional experimentation and high-content analysis. Methods Mol Biol 2013; 1041: 41–51.
Eyupoglu IY, Hahnen E, Heckel A, Siebzehnrubl FA, Buslei R, Fahlbusch R et al. Malignant glioma-induced neuronal cell death in an organotypic glioma invasion model. Technical note. J Neurosurg 2005; 102: 738–744.
Acknowledgements
Our work is supported by the German Research Foundation (DFG Ey 94/2-1).
Author contributions
NES and IYE conceived and designed the study. AG and MAS performed all experiments. MAS and TE contributed to the in vivo experiments and MR imaging analysis under the supervision of AD, MAS, AG, TE, RB and MB analyzed and interpreted the data and discussed the data with all authors. NES and IYE wrote the manuscript. All authors contributed to the preparation of the final manuscript. AG performed the present work in fulfillment of the requirements for obtaining the degree Dr rer. biol. hum. at the Friedrich Alexander University of Erlangen-Nürnberg (FAU).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ghoochani, A., Schwarz, M., Yakubov, E. et al. MIF-CD74 signaling impedes microglial M1 polarization and facilitates brain tumorigenesis. Oncogene 35, 6246–6261 (2016). https://doi.org/10.1038/onc.2016.160
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.160
This article is cited by
-
MIF contribution to progressive brain diseases
Journal of Neuroinflammation (2024)
-
Autologous anti-GD2 CAR T cells efficiently target primary human glioblastoma
npj Precision Oncology (2024)
-
Decoding meningioma heterogeneity and neoplastic cell—macrophage interaction through single-cell transcriptome profiling across pathological grades
Journal of Translational Medicine (2023)
-
Loss of microglial MCT4 leads to defective synaptic pruning and anxiety-like behavior in mice
Nature Communications (2023)
-
Tumor-associated macrophages as a potential therapeutic target in thyroid cancers
Cancer Immunology, Immunotherapy (2023)