Abstract
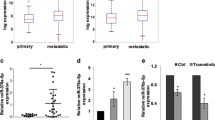
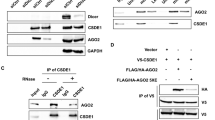
A fundamental event in the development and progression of malignant melanoma is the deregulation of cancer-relevant transcription factors. We recently showed that c-Jun is a main regulator of tumor progression in melanoma and thus the most important member of the AP-1 transcription factor family for this disease. Interestingly, we revealed that c-Jun expression was regulated on the post-transcriptional level and therefore speculated that miRNAs could be involved in c-Jun regulation. We determined seed sequences for miR-125b and miR-527 in the coding region of c-Jun mRNA that hints at the direct involvement of miRNA-dependent regulation on the protein level. We found that the expression of miR-125b was significantly reduced in malignant melanoma cell lines and tissue samples compared with melanocytes, whereas miR-527 remained unchanged. In further functional experiments, treatment of melanoma cells with pre-miR-125b resulted in strong suppression of cellular proliferation and migration, supporting the role of miR-125b in melanoma. In addition, transfection of pre-miR-125b led to strong downregulation of c-Jun protein but not mRNA expression in melanoma cells. Luciferase assays using reporter plasmids containing the miR-125b seed sequence in the luciferase coding region confirmed the direct interaction with miR-125b. Furthermore, immunoprecipitation of Ago-2 revealed that c-Jun mRNA accumulated in the RNA-induced silencing complex after pre-miR-125b transfection in melanoma cells. In summary, we identified an important role for miR-125b in malignant melanoma. Moreover, we demonstrated post-transcriptional regulation of c-Jun by this miRNA and showed that c-Jun is a main mediator of the effects of miR-125b on melanoma cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Eferl R, Wagner EF . AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer 2003; 3: 859–868.
Jochum W, Passegue E, Wagner EF . AP-1 in mouse development and tumorigenesis. Oncogene 2001; 20: 2401–2412.
Weiss C, Bohmann D . Deregulated repression of c-Jun provides a potential link to its role in tumorigenesis. Cell Cycle 2004; 3: 111–113.
Spangler B, Vardimon L, Bosserhoff AK, Kuphal S . Post-transcriptional regulation controlled by E-cadherin is important for c-Jun activity in melanoma. Pigment Cell Melanoma Res 2011; 24: 148–164.
Kovary K, Bravo R . The jun and fos protein families are both required for cell cycle progression in fibroblasts. Mol Cell Biol 1991; 11: 4466–4472.
Shaulian E, Karin M . AP-1 in cell proliferation and survival. Oncogene 2001; 20: 2390–2400.
Gebauer F, Hentze MW . Molecular mechanisms of translational control. Nat Rev Mol Cell Biol 2004; 5: 827–835.
Meister G, Tuschl T . Mechanisms of gene silencing by double-stranded RNA. Nature 2004; 431: 343–349.
Vasudevan S . Posttranscriptional upregulation by MicroRNAs. Wiley Interdiscip Rev RNA 2012; 3: 311–330.
Papaconstantinou I, Karakatsanis A, Gazouli M, Polymeneas G, Voros D . The role of microRNAs in liver cancer. Eur J Gastroenterol Hepatol 2012; 24: 223–228.
Fang YX, Xue JL, Shen Q, Chen J, Tian L . miR-7 inhibits tumor growth and metastasis by targeting the PI3K/AKT pathway in hepatocellular carcinoma. Hepatology 2012; 55: 1852–1862.
Le XF, Merchant O, Bast RC, Calin GA . The roles of micrornas in the cancer invasion-metastasis cascade. Cancer Microenviron 2010; 3: 137–147.
Dalmay T, Edwards DR . MicroRNAs and the hallmarks of cancer. Oncogene 2006; 25: 6170–6175.
Mueller DW, Bosserhoff AK . Role of miRNAs in the progression of malignant melanoma. Br J Cancer 2009; 101: 551–556.
Howell PM, Li X, Riker AI, Xi Y . MicroRNA in melanoma. Ochsner J 2010; 10: 83–92.
Deng Y, Deng H, Bi F, Liu J, Bemis LT, Norris D et al. MicroRNA-137 targets carboxyl-terminal binding protein 1 in melanoma cell lines. Int J Biol Sci 2011; 7: 133–137.
Ender C, Meister G . Argonaute proteins at a glance. J Cell Sci 2010; 123: 1819–1823.
Zeng Y, Yi R, Cullen BR . MicroRNAs and small interfering RNAs can inhibit mRNA expression by similar mechanisms. Proc Natl Acad Sci USA 2003; 100: 9779–9784.
Janga SC, Vallabhaneni S . MicroRNAs as post-transcriptional machines and their interplay with cellular networks. Adv Exp Med Biol 2011; 722: 59–74.
Orom UA, Nielsen FC, Lund AH . MicroRNA-10a binds the 5′UTR of ribosomal protein mRNAs and enhances their translation. Mol Cell 2008; 30: 460–471.
Rigoutsos I . New tricks for animal microRNAS: targeting of amino acid coding regions at conserved and nonconserved sites. Cancer Res 2009; 69: 3245–3248.
Esquela-Kerscher A, Slack FJ . Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer 2006; 6: 259–269.
Chen J, Feilotter HE, Pare GC, Zhang X, Pemberton JG, Garady C et al. MicroRNA-193b represses cell proliferation and regulates cyclin D1 in melanoma. Am J Pathol 2010; 176: 2520–2529.
Xu Y, Brenn T, Brown ER, Doherty V, Melton DW . Differential expression of microRNAs during melanoma progression: miR-200c, miR-205 and miR-211 are downregulated in melanoma and act as tumour suppressors. Br J Cancer 2012; 106: 553–561.
Poser I, Tatzel J, Kuphal S, Bosserhoff AK . Functional role of MIA in melanocytes and early development of melanoma. Oncogene 2004; 23: 6115–6124.
Russo AE, Torrisi E, Bevelacqua Y, Perrotta R, Libra M, McCubrey JA et al. Melanoma: molecular pathogenesis and emerging target therapies (Review). Int J Oncol 2009; 34: 1481–1489.
Shoo BA, Kashani-Sabet M . Melanoma arising in African-, Asian-, Latino- and Native-American populations. Semin Cutan Med Surg 2009; 28: 96–102.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D . Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90.
Mueller DW, Rehli M, Bosserhoff AK . miRNA expression profiling in melanocytes and melanoma cell lines reveals miRNAs associated with formation and progression of malignant melanoma. J Invest Dermatol 2009; 129: 1740–1751.
Philippidou D, Schmitt M, Moser D, Margue C, Nazarov PV, Muller A et al. Signatures of microRNAs and selected microRNA target genes in human melanoma. Cancer Res 2010; 70: 4163–4173.
Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA 2004; 101: 2999–3004.
Glud M, Rossing M, Hother C, Holst L, Hastrup N, Nielsen FC et al. Downregulation of miR-125b in metastatic cutaneous malignant melanoma. Melanoma Res 2010; 20: 479–484.
Glud M, Manfe V, Biskup E, Holst L, Dirksen AM, Hastrup N et al. MicroRNA miR-125b induces senescence in human melanoma cells. Melanoma Res 2011; 21: 253–256.
Poser I, Bosserhoff AK . Transcription factors involved in development and progression of malignant melanoma. Histol Histopathol 2004; 19: 173–188.
Libermann TA, Zerbini LF . Targeting transcription factors for cancer gene therapy. Curr Gene Ther 2006; 6: 17–33.
Spangler B, Kappelmann M, Schittek B, Meierjohann S, Vardimon L, Bosserhoff AK et al. ETS-1/RhoC signaling regulates the transcription factor c-Jun in melanoma. Int J Cancer 2012; 130: 2801–2811.
Friedman RC, Farh KK, Burge CB, Bartel DP . Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 2009; 19: 92–105.
Bartel DP . MicroRNAs: target recognition and regulatory functions. Cell 2009; 136: 215–233.
Katiyar S, Casimiro MC, Dettin L, Ju X, Wagner EF, Tanaka H et al. C-jun inhibits mammary apoptosis in vivo. Mol Biol Cell 2010; 21: 4264–4274.
Braig S, Bosserhoff A-K . Death inducer-obliterator 1 (Dido1) is a BMP target gene and promotes BMP-induced melanoma progression. Oncogene (e-pub ahead of print 2 April 2012; doi:10.1038/onc.2012.115).
Jacob K, Wach F, Holzapfel U, Hein R, Lengyel E, Buettner R et al. In vitro modulation of human melanoma cell invasion and proliferation by all-trans-retinoic acid. Melanoma Res 1998; 8: 211–219.
Polak P, Oren A, Ben Dror I, Steinberg D, Sapoznik S, Arditi-Duvdevany A et al. The cytoskeletal network controls c-Jun translation in a UTR-dependent manner. Oncogene 2006; 25: 665–676.
Rothhammer T, Poser I, Soncin F, Bataille F, Moser M, Bosserhoff AK . Bone morphogenic proteins are overexpressed in malignant melanoma and promote cell invasion and migration. Cancer Res 2005; 65: 448–456.
Braig S, Wallner S, Junglas B, Fuchshofer R, Bosserhoff AK . CTGF is overexpressed in malignant melanoma and promotes cell invasion and migration. Br J Cancer 2011; 105: 231–238.
Schmid R, Schiffner S, Opolka A, Grassel S, Schubert T, Moser M et al. Enhanced cartilage regeneration in MIA/CD-RAP deficient mice. Cell Death Dis 2010; 1: e97.
Acknowledgements
We would like to thank Lisa Ellmann for continuous assistance in cultivation. This work was supported by Melanoma Research Network of the Deutsche Krebshilfe e.V. (German Cancer Aid) and the DFG. GM is supported by grants from the Bavarian Ministry for education and science (BayGene), the European Union (ERC starting grant ‘sRNAs’, FP7 project ‘ONCOMIRs’) and the Bundesministerium für Bildung und Forschung (BMBF, NGFN+).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kappelmann, M., Kuphal, S., Meister, G. et al. MicroRNA miR-125b controls melanoma progression by direct regulation of c-Jun protein expression. Oncogene 32, 2984–2991 (2013). https://doi.org/10.1038/onc.2012.307
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.307
Keywords
This article is cited by
-
Tumour-derived extracellular vesicle based vaccines for melanoma treatment
Drug Delivery and Translational Research (2023)
-
BRAFi induced demethylation of miR-152-5p regulates phenotype switching by targeting TXNIP in cutaneous melanoma
Apoptosis (2020)
-
C-Jun drives melanoma progression in PTEN wild type melanoma cells
Cell Death & Disease (2019)
-
Downregulation of intratumoral expression of miR-205, miR-200c and miR-125b in primary human cutaneous melanomas predicts shorter survival
Scientific Reports (2018)
-
Predict MiRNA-Disease Association with Collaborative Filtering
Neuroinformatics (2018)