Abstract
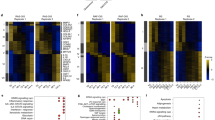
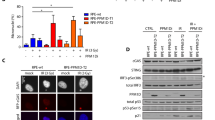
Cellular senescence is a programme of irreversible cell cycle arrest that normal cells undergo in response to progressive shortening of telomeres, changes in telomeric structure, oncogene activation or oxidative stress. The underlying signalling pathways, of major clinicopathological relevance, are unknown. We combined genome-wide expression profiling with genetic complementation to identify genes that are differentially expressed when conditionally immortalised human fibroblasts undergo senescence upon activation of the p16-pRB and p53-p21 tumour suppressor pathways. This identified 816 up and 961 downregulated genes whose expression was reversed when senescence was bypassed. Overlay of this data set with the meta-signatures of genes upregulated in cancer showed that nearly 50% of them were downregulated upon senescence showing that even though overcoming senescence may only be one of the events required for malignant transformation, nearly half of the genes upregulated in cancer are related to it. Moreover 65 of the up and 26 of the downregulated genes are known downstream targets of nuclear factor (NF)-κB suggesting that senescence was associated with activation of the NF-κB pathway. Direct perturbation of this pathway bypasses growth arrest indicating that activation of NF-κB signalling has a causal role in promoting senescence.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Adams PD . (2009). Healing and hurting: molecular mechanisms, functions, and pathologies of cellular senescence. Mol Cell 36: 2–14.
Adler AS, Sinha S, Kawahara TL, Zhang JY, Segal E, Chang HY . (2007). Motif module map reveals enforcement of aging by continual NF-kappaB activity. Genes Dev 21: 3244–3257.
Aggarwal BB, Totpal K, LaPushin R, Chaturvedi MM, Pereira-Smith OM, Smith JR . (1995). Diminished responsiveness of senescent normal human fibroblasts to TNF-dependent proliferation and interleukin production is not due to its effect on the receptors or on the activation of a nuclear factor NF-kappa B. Exp Cell Res 218: 381–388.
Barsotti AM, Prives C . (2009). Pro-proliferative FoxM1 is a target of p53-mediated repression. Oncogene 28: 4295–4305.
Batsi C, Markopoulou S, Vartholomatos G, Georgiou I, Kanavaros P, Gorgoulis VG et al. (2009). Chronic NF-kappaB activation delays RasV12-induced premature senescence of human fibroblasts by suppressing the DNA damage checkpoint response. Mech Ageing Dev 130: 409–419.
Berns K, Hijmans EM, Mullenders J, Brummelkamp TR, Velds A, Heimerikx M et al. (2004). A large-scale RNAi screen in human cells identifies new components of the p53 pathway. Nature 428: 431–437.
Ben-Porath I, Weinberg RA . (2005). The signals and pathways activating cellular senescence. Int J Biochem Cell Biol 37: 961–976.
Besnier C, Ylinen L, Strange B, Lister A, Takeuchi Y, Goff SP et al. (2003). Characterization of murine leukemia virus restriction in mammals. J Virol 77: 13403–13406.
Campisi J, d'Adda di Fagagna F . (2007). Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8: 729–740.
Collado M, Blasco MA, Serrano M . (2007). Cellular senescence in cancer and aging. Cell 130: 223–233.
Counter CM, Hahn WC, Wei W, Caddle SD, Beijersbergen RL, Lansdorp PM et al. (1998). Dissociation among in vitro telomerase activity, telomere maintenance, and cellular immortalization. Proc Natl Acad Sci USA 95: 14723–14728.
Dimri GP, Campisi J . (1994). Altered profile of transcription factor-binding activities in senescent human fibroblasts. Exp Cell Res 212: 132–140.
Dodeller F, Gottar M, Huesken D, Iourgenko V, Cenni B . (2008). The lysosomal transmembrane protein 9B regulates the activity of inflammatory signaling pathways. J Biol Chem 283: 21487–21494.
Gonos ES, Burns JS, Mazars GR, Kobrna A, Riley TE, Barnett SC et al. (1996). Rat embryo fibroblasts immortalized with simian virus 40 large T antigen undergo senescence upon its inactivation. Mol Cell Biol 16: 5127–5138.
Gorman SD, Cristofalo VJ . (1985). Reinitiation of cellular DNA synthesis in BrdU-selected nondividing senescent WI-38 cells by simian virus 40 infection. J Cell Physiol 125: 122–126.
Hanahan D, Weinberg RA . (2000). The hallmarks of cancer. Cell 100: 57–70.
Hardy K, Mansfield L, Mackay A, Benvenuti S, Ismail S, Arora P et al. (2005). Transcriptional networks and cellular senescence in human mammary fibroblasts. Mol Biol Cell 16: 943–953.
Hayden MS, Ghosh S . (2008). Shared principles in NF-kappaB signaling. Cell 132: 344–362.
Hayflick L, Moorhead PS . (1961). The serial cultivation of human diploid cell strains. Exp Cell Res 25: 585–621.
He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y et al. (2007). A microRNA component of the p53 tumour suppressor network. Nature 447: 1130–1134.
Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U et al. (2003). Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4: 249–264.
Jensen LJ, Kuhn M, Stark M, Chaffron S, Creevey C, Muller J et al. (2009). STRING 8—a global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res 37: D412–D416.
Karin M . (2009). NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol 1: a000141.
Kiyono T, Foster SA, Koop JI, McDougall JK, Galloway DA, Klingelhutz AJ . (1998). Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 396: 84–88.
Kriete A, Mayo KL, Yalamanchili N, Beggs W, Bender P, Kari C et al. (2008). Cell autonomous expression of inflammatory genes in biologically aged fibroblasts associated with elevated NF-kappaB activity. Immun Ageing 5: 5.
Kriete A, Mayo KL . (2009). Atypical pathways of NF-kappaB activation and aging. Exp Gerontol 44: 250–255.
Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ et al. (2008). Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133: 1019–1031.
Kuilman T, Peeper DS . (2009). Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer 9: 81–94.
Laoukili J, Alvarez M, Meijer LA, Stahl M, Mohammed S, Kleij L et al. (2008a). Activation of FoxM1 during G2 requires cyclin A/Cdk-dependent relief of autorepression by the FoxM1 N-terminal domain. Mol Cell Biol 28: 3076–3087.
Laoukili J, Alvarez-Fernandez M, Stahl M, Medema RH . (2008b). FoxM1 is degraded at mitotic exit in a Cdh1-dependent manner. Cell Cycle 7: 2720–2726.
Laoukili J, Stahl M, Medema RH . (2007). FoxM1: at the crossroads of ageing and cancer. Biochim Biophys Acta 1775: 92–102.
Ly DH, Lockhart DJ, Lerner RA, Schultz PG . (2000). Mitotic misregulation and human aging. Science 287: 2486–2492.
Mansfield LV . (2006). Dissecting the Telomere–Independent Pathways Underlying Human Cellular Senescence, PhD thesis. University of London, London, UK.
Matsuda A, Suzuki Y, Honda G, Muramatsu S, Matsuzaki O, Nagano Y et al. (2003). Large-scale identification and characterization of human genes that activate NF-kappaB and MAPK signaling pathways. Oncogene 22: 3307–3318.
Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J . (2001). NF-kappaB signals induce the expression of c-FLIP. Mol Cell Biol 21: 5299–5305.
Momand J, Zambetti GP, Olson DC, George D, Levine AJ . (1992). The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell 69: 1237–1245.
O'Hare MJ, Bond J, Clarke C, Takeuchi Y, Atherton AJ, Berry C et al. (2001). Conditional immortalization of freshly isolated human mammary fibroblasts and endothelial cells. Proc Natl Acad Sci USA 98: 646–651.
Orjalo AV, Bhaumik D, Gengler BK, Scott GK, Campisi J . (2009). Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc Natl Acad Sci USA 106: 17031–17036.
Ossovskaya VS, Mazo IA, Chernov MV, Chernova OB, Strezoska Z, Kondratov R et al. (1996). Use of genetic suppressor elements to dissect distinct biological effects of separate p53 domains. Proc Natl Acad Sci USA 93: 10309–10314.
Park HJ, Wang Z, Costa RH, Tyner A, Lau LF, Raychaudhuri P . (2008). An N-terminal inhibitory domain modulates activity of FoxM1 during cell cycle. Oncogene 27: 1696–1704.
Passos JF, Nelson G, Wang C, Richter T, Simillion C, Proctor CJ et al. (2010). Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol 6: 347.
Penzo M, Massa PE, Olivotto E, Bianchi F, Borzi RM, Hanidu A et al. (2009). Sustained NF-kappaB activation produces a short-term cell proliferation block in conjunction with repressing effectors of cell cycle progression controlled by E2F or FoxM1. J Cell Physiol 218: 215–227.
Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S et al. (2004). NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 431: 461–466.
Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D et al. (2004). Large-scale meta-analysis of cancer microarray data identifies common transcriptional profiles of neoplastic transformation and progression. Proc Natl Acad Sci USA 101: 9309–9314.
Rowland BD, Denissov SG, Douma S, Stunnenberg HG, Bernards R, Peeper DS . (2002). E2F transcriptional repressor complexes are critical downstream targets of p19 (ARF)/p53-induced proliferative arrest. Cancer Cell 2: 55–65.
Salminen A, Huuskonen J, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T . (2008). Activation of innate immunity system during aging: NF-kB signalling is the molecular culprit of inflamm-aging. Ageing Res Rev 7: 83–105.
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D et al. (2003). Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13: 2498–2504.
Shelton DN, Chang E, Whittier PS, Choi D, Funk WD . (1999). Microarray analysis of replicative senescence. Curr Biol 9: 939–945.
Taganov KD, Boldin MP, Chang KJ, Baltimore D . (2006). NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA 103: 12481–12486.
Walia V, Ding M, Kumar S, Nie D, Premkumar LS, Elble RC . (2009). hCLCA2 is a p53-inducible inhibitor of breast cancer cell proliferation. Cancer Res 69: 6624–6632.
Wang IC, Chen YJ, Hughes D, Petrovic V, Major ML, Park HJ et al. (2005). Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Mol Cell Biol 25: 10875–10894.
Whitfield ML, Sherlock G, Saldanha AJ, Murray JI, Ball CA, Alexander KE et al. (2002). Identification of genes periodically expressed in the human cell cycle and their expression in tumors. Mol Biol Cell 13: 1977–2000.
Yamakuchi M, Ferlito M, Lowenstein CJ . (2008). miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci USA 105: 13421–13426.
Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA et al. (2004). Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23: 2369–2380.
Acknowledgements
We are indebted to AM Neville, A Baldwin, P Meier, M Resnicoff and N Perkins for advice, helpful discussions and critical reading of the manuscript. We thank J Downward, X Lu, P Meier, G Towers and C King (the UCL shRNA library core facility) for providing reagents. We are grateful to E Ortenberg and J White of Biotrove Inc. for the qPCR analysis, S Shah and A Grigoriadis for bioinformatic analysis and R Young for graphics. PSJ gratefully acknowledges financial support from the Wellcome Trust (078305) and an equipment grant from the Brain Research Trust.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Rovillain, E., Mansfield, L., Caetano, C. et al. Activation of nuclear factor-kappa B signalling promotes cellular senescence. Oncogene 30, 2356–2366 (2011). https://doi.org/10.1038/onc.2010.611
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2010.611
Keywords
This article is cited by
-
Colchicine prevents oxidative stress-induced endothelial cell senescence via blocking NF-κB and MAPKs: implications in vascular diseases
Journal of Inflammation (2023)
-
Common factors among three types of cells aged in mice
Biogerontology (2023)
-
NF-κB is a critical mediator of post-mitotic senescence in oligodendrocytes and subsequent white matter loss
Molecular Neurodegeneration (2023)
-
In vivo cyclic induction of the FOXM1 transcription factor delays natural and progeroid aging phenotypes and extends healthspan
Nature Aging (2022)
-
Senolytic Effect of Cerium Oxide Nanoparticles (CeO2 NPs) by Attenuating p38/NF-кB, and p53/p21 Signaling Pathways
Journal of Cluster Science (2022)