Abstract
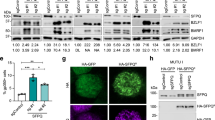
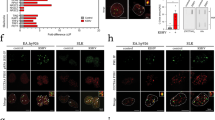
Epstein–Barr virus (EBV) has been implicated in the pathogenesis of human malignancies, but its contribution to tumorigenesis is not well understood. EBV carriage is associated with increased genomic instability in Burkitt's lymphoma, suggesting that viral products may induce this tumor phenotype. Using a panel of transfected sublines of the B-lymphoma line BJAB expressing the viral genes associated with latent infection, we show that the EBV nuclear antigens, EBNA-1 and EBNA-3C, and the latent membrane protein 1, LMP-1, independently promote genomic instability, as detected by nonclonal chromosomal aberrations, DNA breaks and phosphorylation of histone H2AX. EBNA-1 promotes the generation of DNA damage by inducing reactive oxygen species (ROS), whereas DNA repair is inhibited in LMP-1-expressing cells through downregulation of the DNA damage-sensing kinase, ataxia telangiectasia mutated (ATM), reduction of phosphorylation of its downstream targets Chk2 and inactivation of the G2 checkpoint. EBNA-3C enhances the propagation of damaged DNA through inactivation of the mitotic spindle checkpoint and transcriptional downregulation of BubR1. Thus, multiple cellular functions involved in the maintenance of genome integrity seem to be independently targeted by EBV, pointing to the induction of genomic instability as a critical event in viral oncogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Åman P, Rowe M, Kai C, Finke J, Rymo L, Klein E et al. (1990). Effect of the EBNA-2 gene on the surface antigen phenotype of transfected EBV-negative B-lymphoma lines. Int J Cancer 45: 77–82.
Bolt J, Vo QN, Kim WJ, McWhorter AJ, Thomson J, Hagensee ME et al. (2005). The ATM/p53 pathway is commonly targeted for inactivation in squamous cell carcinoma of the head and neck (SCCHN) by multiple molecular mechanisms. Oral Oncol 41: 1013–1020.
Bose S, Starczynski J, Chukwuma M, Baumforth K, Wei W, Morgan S et al. (2007). Down-regulation of ATM protein in HRS cells of nodular sclerosis Hodgkin's lymphoma in children occurs in the absence of ATM gene inactivation. J Pathol 213: 329–336.
Bose S, Yap LF, Fung M, Starzcynski J, Saleh A, Morgan S et al. (2009). The ATM tumour suppressor gene is down-regulated in EBV-associated nasopharyngeal carcinoma. J Pathol 217: 345–352.
Chandel NS, Schumacker PT, Arch RH . (2001). Reactive oxygen species are downstream products of TRAF-mediated signal transduction. J Biol Chem 276: 42728–42736.
Chaturvedi P, Eng WK, Zhu Y, Mattern MR, Mishra R, Hurle MR et al. (1999). Mammalian Chk2 is a downstream effector of the ATM-dependent DNA damage checkpoint pathway. Oncogene 18: 4047–4054.
Chen YR, Liu MT, Chang YT, Wu CC, Hu CY, Chen JY . (2008). Epstein-Barr virus latent membrane protein 1 represses DNA repair through the PI3K/Akt/FOXO3a pathway in human epithelial cells. J Virol 82: 8124–8137.
Choudhuri T, Verma SC, Lan K, Murakami M, Robertson ES . (2007). The ATM/ATR signaling effector Chk2 is targeted by Epstein-Barr virus nuclear antigen 3C to release the G2/M cell cycle block. J Virol 81: 6718–6730.
Cuomo L, Trivedi P, Wang F, Winberg G, Klein G, Masucci MG . (1990). Expression of the Epstein-Barr virus (EBV)-encoded membrane antigen (LMP) increases the stimulatory capacity of EBV-negative B lymphoma lines in allogeneic mixed lymphocyte cultures. Eur J Immunol 20: 2293–2299.
Eclache V, Caulet-Maugendre S, Poirel HA, Djemai M, Robert J, Lejeune F et al. (2004). Cryptic deletion involving the ATM locus at 11q22.3 approximately q23.1 in B-cell chronic lymphocytic leukemia and related disorders. Cancer Genet Cytogenet 152: 72–76.
Erickson KD, Martin JM . (2000). The late lytic LMP-1 protein of Epstein-Barr virus can negatively regulate LMP-1 signaling. J Virol 74: 1057–1060.
Floettmann JE, Ward K, Rickinson AB, Rowe M . (1996). Cytostatic effect of Epstein-Barr virus latent membrane protein-1 analyzed using tetracycline-regulated expression in B cell lines. Virology 223: 29–40.
Greene LM, Campiani G, Lawler M, Williams DC, Zisterer DM . (2008). BubR1 is required for a sustained mitotic spindle checkpoint arrest in human cancer cells treated with tubulin-targeting pyrrolo-1,5-benzoxazepines. Mol Pharmacol 73: 419–430.
Gruhne B, Sompallae R, Marescotti D, Kamranvar SA, Gastaldello S, Masucci MG . (2009). The Epstein-Barr virus nuclear antigen-1 promotes genomic instability via induction of reactive oxygen species. Proc Natl Acad Sci USA 106: 2313–2318.
Hudson GS, Farrell PJ, Barrell BG . (1985). Two related but differentially expressed potential membrane proteins encoded by the EcoRI Dhet region of Epstein-Barr virus B95-8. J Virol 53: 528–535.
Huggett J, Dheda K, Bustin S, Zumla A . (2005). Real-time RT-PCR normalisation; strategies and considerations. Genes Immun 6: 279–284.
Iliakis G, Wang Y, Guan J, Wang H . (2003). DNA damage checkpoint control in cells exposed to ionizing radiation. Oncogene 22: 5834–5847.
Kamranvar SA, Gruhne B, Szeles A, Masucci MG . (2007). Epstein-Barr virus promotes genomic instability in Burkitt's lymphoma. Oncogene 26: 5115–5123.
Kang MS, Hung SC, Kieff E . (2001). Epstein-Barr virus nuclear antigen 1 activates transcription from episomal but not integrated DNA and does not alter lymphocyte growth. Proc Natl Acad Sci USA 98: 15233–15238.
Kelly G, Bell A, Rickinson A . (2002). Epstein-Barr virus-associated Burkitt lymphomagenesis selects for downregulation of the nuclear antigen EBNA2. Nat Med 8: 1098–1104.
Kelly GL, Milner AE, Baldwin GS, Bell AI, Rickinson AB . (2006). Three restricted forms of Epstein-Barr virus latency counteracting apoptosis in c-myc-expressing Burkitt lymphoma cells. Proc Natl Acad Sci USA 103: 14935–14940.
Klein G . (1986). Constitutive activation of oncogenes by chromosomal translocations in B-cell derived tumors. AIDS Res 2 (Suppl 1): S167–S176.
Knight JS, Robertson ES . (2004). Epstein-Barr virus nuclear antigen 3C regulates cyclin A/p27 complexes and enhances cyclin A-dependent kinase activity. J Virol 78: 1981–1991.
Knight JS, Sharma N, Robertson ES . (2005). Epstein-Barr virus latent antigen 3C can mediate the degradation of the retinoblastoma protein through an SCF cellular ubiquitin ligase. Proc Natl Acad Sci USA 102: 18562–18566.
Kulukian A, Han JS, Cleveland DW . (2009). Unattached kinetochores catalyze production of an anaphase inhibitor that requires a Mad2 template to prime Cdc20 for BubR1 binding. Dev Cell 16: 105–117.
Kulwichit W, Edwards RH, Davenport EM, Baskar JF, Godfrey V, Raab-Traub N . (1998). Expression of the Epstein-Barr virus latent membrane protein 1 induces B cell lymphoma in transgenic mice. Proc Natl Acad Sci USA 95: 11963–11968.
Leao M, Anderton E, Wade M, Meekings K, Allday MJ . (2007). Epstein-Barr virus-induced resistance to drugs that activate the mitotic spindle assembly checkpoint in Burkitt's lymphoma cells. J Virol 81: 248–260.
Liu MT, Chang YT, Chen SC, Chuang YC, Chen YR, Lin CS et al. (2005). Epstein-Barr virus latent membrane protein 1 represses p53-mediated DNA repair and transcriptional activity. Oncogene 24: 2635–2646.
Liu MT, Chen YR, Chen SC, Hu CY, Lin CS, Chang YT et al. (2004). Epstein-Barr virus latent membrane protein 1 induces micronucleus formation, represses DNA repair and enhances sensitivity to DNA-damaging agents in human epithelial cells. Oncogene 23: 2531–2539.
Marshall D, Sample C . (1995). Epstein-Barr virus nuclear antigen 3C is a transcriptional regulator. J Virol 69: 3624–3630.
McIntosh JR . (1991). Structural and mechanical control of mitotic progression. Cold Spring Harb Symp Quant Biol 56: 613–619.
Menezes J, Leibold W, Klein G, Clements G . (1975). Establishment and characterization of an Epstein-Barr virus (EBC)-negative lymphoblastoid B cell line (BJA-B) from an exceptional, EBV-genome-negative African Burkitt's lymphoma. Biomedicine 22: 276–284.
Mosialos G, Birkenbach M, Yalamanchili R, VanArsdale T, Ware C, Kieff E . (1995). The Epstein-Barr virus transforming protein LMP1 engages signaling proteins for the tumor necrosis factor receptor family. Cell 80: 389–399.
Nakamura N, Ramaswamy S, Vazquez F, Signoretti S, Loda M, Sellers WR . (2000). Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol Cell Biol 20: 8969–8982.
Pan SH, Tai CC, Lin CS, Hsu WB, Chou SF, Lai CC et al. (2009). Epstein-Barr virus nuclear antigen 2 disrupts mitotic checkpoint and causes chromosomal instability. Carcinogenesis 30: 366–375.
Parish CR, Glidden MH, Quah BJ, Warren HS . (2009). Use of the intracellular fluorescent dye CFSE to monitor lymphocyte migration and proliferation. Curr Protoc Immunol (Chapter 4, Unit 4.9).
Pfeffer S, Zavolan M, Grasser FA, Chien M, Russo JJ, Ju J et al. (2004). Identification of virus-encoded microRNAs. Science 304: 734–736.
Ranjan D, Siquijor A, Johnston TD, Wu G, Nagabhuskahn M . (1998). The effect of curcumin on human B-cell immortalization by Epstein-Barr virus. Am Surg 64: 47–51; discussion 51–2.
Rao CV, Yang YM, Swamy MV, Liu T, Fang Y, Mahmood R et al. (2005). Colonic tumorigenesis in BubR1+/-ApcMin/+ compound mutant mice is linked to premature separation of sister chromatids and enhanced genomic instability. Proc Natl Acad Sci USA 102: 4365–4370.
Raptis S, Bapat B . (2006). Genetic instability in human tumors. EXS 96: 303–320.
Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM . (1998). DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 273: 5858–5868.
Rowe DT, Rowe M, Evan GI, Wallace LE, Farrell PJ, Rickinson AB . (1986). Restricted expression of EBV latent genes and T-lymphocyte-detected membrane antigen in Burkitt's lymphoma cells. EMBO J 5: 2599–2607.
Saha A, Murakami M, Kumar P, Bajaj B, Sims K, Robertson ES . (2009). Epstein Barr virus nuclear antigen 3C augments Mdm2 mediated p53 ubiquitination and degradation by deubiquitinating Mdm2. J Virol 83: 4652–4669.
Schaefer BC, Strominger JL, Speck SH . (1995). Redefining the Epstein-Barr virus-encoded nuclear antigen EBNA-1 gene promoter and transcription initiation site in group I Burkitt lymphoma cell lines. Proc Natl Acad Sci USA 92: 10565–10569.
Shea LM, Beehler C, Schwartz M, Shenkar R, Tuder R, Abraham E . (1996). Hyperoxia activates NF-kappaB and increases TNF-alpha and IFN-gamma gene expression in mouse pulmonary lymphocytes. J Immunol 157: 3902–3908.
Sivachandran N, Sarkari F, Frappier L . (2008). Epstein-Barr nuclear antigen 1 contributes to nasopharyngeal carcinoma through disruption of PML nuclear bodies. PLoS Pathog 4: e1000170.
Stollmann B, Fonatsch C, Havers W . (1985). Persistent Epstein-Barr virus infection associated with monosomy 7 or chromosome 3 abnormality in childhood myeloproliferative disorders. Br J Haematol 60: 183–196.
Taylor AM, Metcalfe JA, Thick J, Mak YF . (1996). Leukemia and lymphoma in ataxia telangiectasia. Blood 87: 423–438.
Tran H, Brunet A, Grenier JM, Datta SR, Fornace Jr AJ, DiStefano PS et al. (2002). DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science 296: 530–534.
Wang F, Gregory C, Sample C, Rowe M, Liebowitz D, Murray R et al. (1990). Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J Virol 64: 2309–2318.
Winberg G, Matskova L, Chen F, Plant P, Rotin D, Gish G et al. (2000). Latent membrane protein 2A of Epstein-Barr virus binds WW domain E3 protein-ubiquitin ligases that ubiquitinate B-cell tyrosine kinases. Mol Cell Biol 20: 8526–8535.
Yates JL, Warren N, Sugden B . (1985). Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. Nature 313: 812–815.
Young LS, Dawson CW, Clark D, Rupani H, Busson P, Tursz T et al. (1988). Epstein-Barr virus gene expression in nasopharyngeal carcinoma. J Gen Virol 69 (Pt 5): 1051–1065.
Young LS, Rickinson AB . (2004). Epstein-Barr virus: 40 years on. Nat Rev Cancer 4: 757–768.
Acknowledgements
We thank L Rymo, M Rowe and J Middledorp for the kind gift of transfected cell lines and antibodies and also many colleagues for helpful discussions. This study was supported by grants awarded by the Swedish Cancer Society, the Swedish Medical Research Council and Karolinska Institutet, Stockholm, Sweden and by the European Community Integrated Project on Infection and Cancer, INCA, Project No. LSHC-CT-2005-018704.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Rights and permissions
About this article
Cite this article
Gruhne, B., Sompallae, R. & Masucci, M. Three Epstein–Barr virus latency proteins independently promote genomic instability by inducing DNA damage, inhibiting DNA repair and inactivating cell cycle checkpoints. Oncogene 28, 3997–4008 (2009). https://doi.org/10.1038/onc.2009.258
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2009.258
Keywords
This article is cited by
-
High plasma EBV-DNA load and positive EBER status associated with viral recurrence and persistent infection in early treatment of lymphoma
Clinical and Experimental Medicine (2022)
-
Co-expression of low-risk HPV E6/E7 and EBV LMP-1 leads to precancerous lesions by DNA damage
BMC Cancer (2021)
-
The Epstein–Barr virus nuclear antigen-1 upregulates the cellular antioxidant defense to enable B-cell growth transformation and immortalization
Oncogene (2020)
-
Integrative microRNA and mRNA deep-sequencing expression profiling in endemic Burkitt lymphoma
BMC Cancer (2017)
-
Limited nucleotide pools restrict Epstein–Barr virus-mediated B-cell immortalization
Oncogenesis (2017)