Abstract
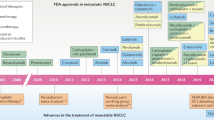
Non-small-cell lung cancer (NSCLC) is a major global health problem and is the leading cause of cancer death worldwide. Current treatment involves nonspecific, nonselective cytotoxic chemotherapy, which results in only a modest increase in survival and causes significant toxicity to the patient. Targeted agents are initially effective in certain small subpopulations of patients, but eventually nearly all patients become resistant to further treatment. The limitations in efficacy and safety associated with available treatments for NSCLC underscore the need for novel agents with improved efficacy and safety profiles. This review discusses the limitations of currently recommended therapies for patients with advanced NSCLC and discusses new agents in clinical development for this disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Abrams TJ, Lee LB, Murray LJ, Pryer NK, Cherrington JM . (2003). SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther 2: 471–478.
Adams JM, Cory S . (2007). The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 26: 1324–1327.
Avastin® (bevacizumab) prescribing information (2008). Genentech, Inc, South San Francisco, CA. Available at: http://www.gene.com/gene/products/information/pdf/avastin-prescribing.pdf. Accessed 19 September 2008.
Balak MN, Gong Y, Riely GJ, Somwar R, Li AR, Zakowski MF et al. (2006). Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res 12: 6494–6501.
Bazley LA, Gullick WJ . (2005). The epidermal growth factor receptor family. Endocr Relat Cancer 12 (Suppl 1): S17–S27.
Bolonaki I, Kotsakis A, Papadimitraki E, Aggouraki D, Konsolakis G, Vagia A et al. (2007). Vaccination of patients with advanced non-small-cell lung cancer with an optimized cryptic human telomerase reverse transcriptase peptide. J Clin Oncol 25: 2727–2734.
Brandes JC, Grossman SA, Ahmad H . (2006). Alteration of pemetrexed excretion in the presence of acute renal failure and effusions: presentation of a case and review of the literature. Cancer Invest 24: 283–287.
Breathnach OS, Freidlin B, Conley B, Green MR, Johnson DH, Gandara DR et al. (2001). Twenty-two years of phase III trials for patients with advanced non-small-cell lung cancer: sobering results. J Clin Oncol 19: 1734–1742.
Britten CD . (2004). Targeting ErbB receptor signaling: a pan-ErbB approach to cancer. Mol Cancer Ther 3: 1335–1342.
Butts CA, Bodkin D, Middleman EL, Englund CW, Ellison D, Alam YZ et al. (2007). Gemcitabine/platinum alone or in combination with cetuximab as first-line treatment for advanced non-small cell lung cancer (NSCLC). J Clin Oncol 25: 394s (ASCO Annual Meeting Proceedings Part I), abstract 7539. Available at http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&conFID=47&abstractID=31939. Accessed 22 July 2009.
Campos L, Rouault JP, Sabido O, Oriol P, Roubi N, Vasselon C et al. (1993). High expression of bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood 81: 3091–3096.
Carney DN . (2002). Lung cancer—time to move on from chemotherapy. N Engl J Med 346: 126–128.
Clark GM, Zborowski DM, Santabarbara P, Ding K, Whitehead M, Seymour L et al. (2006). Smoking history and epidermal growth factor receptor expression as predictors of survival benefit from erlotinib for patients with non-small-cell lung cancer in the National Cancer Institute of Canada Clinical Trials Group study BR.21. Clin Lung Cancer 7: 389–394.
Crawford J, Dale DC, Lyman GH . (2004). Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer 100: 228–237.
Dole M, Nuñez G, Merchant AK, Maybaum J, Rode CK, Bloch CA et al. (1994). Bcl-2 inhibits chemotherapy-induced apoptosis in neuroblastoma. Cancer Res 54: 3253–3259.
Dubey S, Brown RL, Esmond SL, Bowers BJ, Healy JM, Schiller JH . (2005). Patient preferences in choosing chemotherapy regimens for advanced non-small cell lung cancer. J Support Oncol 3: 149–154.
Eberhard DA, Johnson BE, Amler LC, Goddard AD, Heldens SL, Herbst RS et al. (2005). Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol 23: 5900–5909.
Elkind NB, Szentpétery Z, Apáti A, Ozvegy-Laczka C, Várady G, Ujhelly O et al. (2005). Multidrug transporter ABCG2 prevents tumor cell death induced by the epidermal growth factor receptor inhibitor Iressa (ZD1839, Gefitinib). Cancer Res 65: 1770–1777.
Engelman JA, Jänne PA . (2008). Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res 14: 2895–2899.
Engelman JA, Zejnullahu K, Gale CM, Lifshits E, Gonzales AJ, Shimamura T et al. (2007). PF00299804, an irreversible pan-ErbB inhibitor, is effective in lung cancer models with EGFR and ErbB2 mutations that are resistant to gefitinib. Cancer Res 67: 11924–11932.
Eskens F, Mom CH, Planting AST, Gietema JA, Amelsberg A, Huisman H et al. (2008). A phase I dose escalation study of BIBW 2992, an irreversible dual inhibitor of epidermal growth factor receptor 1 (EGFR) and 2 (HER2) tyrosine kinase in a 2-week on, 2-week off schedule in patients with advanced solid tumours. Br J Cancer 98: 80–85.
Gatzemeier U, Blumenschein G, Fosella F, Simantov R, Elting J, Bigwood D et al. (2006). Phase II trial of single-agent sorafenib in patients with advanced non-small cell lung carcinoma. J Clin Oncol 24: (ASCO Annual Meeting Proceedings Part I), abstract 7001.
Gendreau SB, Ventura R, Keast P, Laird AD, Yakes FM, Zhang W et al. (2007). Inhibition of the T790M gatekeeper mutant of the epidermal growth factor receptor by EXEL-7647. Clin Cancer Res 13: 3713–3723.
Greulich H, Chen TH, Feng W, Jänne PA, Alvarez JV, Zappaterra M et al. (2005). Oncogenic transformation by inhibitor-sensitive and -resistant EGFR mutants. PLoS Med 2: e313.
Gridelli C, Maione P, Del Gaizo F, Colantuoni G, Guerriero C, Ferrara C et al. (2007). Sorafenib and sunitinib in the treatment of advanced non-small cell lung cancer. Oncologist 12: 191–200.
Haber DA, Settleman J . (2005). Overcoming acquired resistance to Iressa/Tarceva with inhibitors of a different class. Cell Cycle 4: 1057–1059.
Habib AA, Chun SJ, Neel BG, Vartanian T . (2003). Increased expression of epidermal growth factor receptor induces sequestration of extracellular signal-related kinases and selective attenuation of specific epidermal growth factor-mediated signal transduction pathways. Mol Cancer Res 1: 219–233.
Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J et al. (2004). Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 22: 1589–1597.
Herbst RS, Ansari RH, Gorbunova V, Manikhas G, Saleh M, Boyd T et al. (2007). Multicenter, randomized study of Docetaxel versus Docetaxel plus Oblimersen in patients previously treated for non-small cell lung cancer (NSCLC): B1-06. J Thorac Oncol 2: S335.
Herbst RS, Frankel SR . (2004). Oblimersen sodium (Genasense bcl-2 antisense oligonucleotide): a rational therapeutic to enhance apoptosis in therapy of lung cancer. Clin Cancer Res 10: 4245S–4248S.
Heymach JV, Paz-Ares L, De Braud F, Sebastian M, Stewart DJ, Eberhardt WE et al. (2007). Randomized phase II study of vandetanib (VAN) alone or in combination with carboplatin and paclitaxel (CP) as first-line treatment for advanced non-small-cell lung cancer (NSCLC). J Clin Oncol 25 (Suppl): abstract no. 7544 395s.
Hilberg F, Roth GJ, Krssak M, Kautschitsch S, Sommergruber W, Tontsch-Grunt U et al. (2008). BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res 68: 4774–4782.
Hirsch FR, Varella-Garcia M, Cappuzzo F, McCoy J, Bemis L, Xavier AC et al. (2007). Combination of EGFR gene copy number and protein expression predicts outcome for advanced non-small-cell lung cancer patients treated with gefitinib. Ann Oncol 18: 752–760.
Jackman DM, Yeap BY, Lindeman NI, Fidias P, Rabin MS, Temel J et al. (2007). Phase II clinical trial of chemotherapy-naive patients > or =70 years of age treated with erlotinib for advanced non-small-cell lung cancer. J Clin Oncol 25: 760–766.
Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM et al. (2004). Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 22: 2184–2191.
Kelly K, Crowley J, Bunn Jr PA, Presant CA, Grevstad PK, Moinpour CM et al. (2001). Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non-small-cell lung cancer: a Southwest Oncology Group trial. J Clin Oncol 19: 3210–3218.
Kobayashi S, Boggon TJ, Dayaram T, Jänne PA, Kocher O, Meyerson M et al. (2005). EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 352: 786–792.
Kosaka T, Yatabe Y, Endoh H, Yoshida K, Hida T, Tsuboi M et al. (2006). Analysis of epidermal growth factor receptor gene mutation in patients with non-small cell lung cancer and acquired resistance to gefitinib. Clin Cancer Res 12: 5764–5769.
Kwak EL, Sordella R, Bell DW, Godin-Heymann N, Okimoto RA, Brannigan BW et al. (2005). Irreversible inhibitors of the EGFR receptor may circumvent acquired resistance to gefitinib. Proc Natl Acad Sci USA 102: 7665–7670.
Maheswaran S, Sequist LV, Nagrath S, Ulkus L, Brannigan B, Collura CV et al. (2008). Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med 359: 366–377.
Manegold C, Gravenor D, Woytowitz D, Mezger J, Hirsh V, Albert G et al. (2008). Randomized phase II trial of a toll-like receptor 9 agonist oligodeoxynucleotide, PF-3512676, in combination with first-line taxane plus platinum chemotherapy for advanced-stage non-small-cell lung cancer. J Clin Oncol 26: 3979–3986.
Marks PA, Richon VM, Rifkind RA . (2000). Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. J Natl Cancer Inst 92: 1210–1216.
Massarelli E, Varella-Garcia M, Tang X, Xavier AC, Ozburn NC, Liu DD et al. (2007). KRAS mutation is an important predictor of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. Clin Cancer Res 13: 2890–2896.
Metro G, Finocchiaro G, Cappuzzo F . (2006). Anti-cancer therapy with EGFR inhibitors: factors of prognostic and predictive significance. Ann Oncol 17 (Suppl 2): ii42–ii45.
Miller VA, Wakelee HA, Lara PN, Cho J, Chowhan NM, Pennell I et al. (2008). Activity and tolerance of XL647 in NSCLC patients with acquired resistance to EGFR-TKIs: preliminary results of a phase II trial. J Clin Oncol 26 (Suppl): abstract 8028.
Mita AC, Sweeney CJ, Baker SD, Goetz A, Hammond LA, Patnaik A et al. (2006). Phase I and pharmacokinetic study of pemetrexed administered every 3 weeks to advanced cancer patients with normal and impaired renal function. J Clin Oncol 24: 552–562.
Morgillo F, Kim WY, Kim ES, Ciardiello F, Hong WK, Lee HY . (2007). Implication of the insulin-like growth factor-IR pathway in the resistance of non-small cell lung cancer cells to treatment with gefitinib. Clin Cancer Res 13: 2795–2803.
Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM et al. (2005). A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med 3: 9.
Mross KB, Gmehling D, Frost A, Baas F, Strecker R, Hennig J et al. (2005). A clinical, phase I pharmacokinetic (PK) and pharmacodynamic study of twice-daily BIBF 1120 in advanced cancer patients. J Clin Oncol 23 (Suppl 16): abstract 3031.
Natale RB, Bodkin D, Govindan R, Sleckman B, Rizvi N, Capo A et al. (2006). ZD6474 versus gefitinib in patients with advanced NSCLC: final results from a two-part, double-blind, randomized phase II trial. J Clin Oncol 24 (Suppl): abstract no. 7000 364s.
National Comprehensive Cancer Network (2008). Clinical Practice Guidelines: Non-small Cell Lung Cancer v.2. Available at: http://www.nccn.org/professionals/physician_gls/PDF/nscl.pdf. Accessed 19 September 2008.
Nemunaitis J, Dillman RO, Schwarzenberger PO, Senzer N, Cunningham C, Cutler J et al. (2006). Phase II study of Lucanix™ a transforming growth factor β2 (TGF-β2) antisense gene modified allogeneic tumor cell vaccine in non small cell lung cancer. Mol Ther 13 (Suppl 1): S424–S425; abstract no. 1104.
O’Brien ME, Anderson H, Kaukel E, O'Byrne K, Pawlicki M, Von Pawel J et al. (2004). SRL172 (killed Mycobacterium vaccae) in addition to standard chemotherapy improves quality of life without affecting survival, in patients with advanced non-small-cell lung cancer: phase III results. Ann Oncol 15: 906–914.
Ohe Y, Ohashi Y, Kubota K, Tamura T, Nakagawa K, Negoro S et al. (2007). Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: four-Arm Cooperative Study in Japan. Ann Oncol 18: 317–323.
Pao W, Wang TY, Riely GJ, Miller VA, Pan Q, Ladanyi M et al. (2005). KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med 2: e17.
Pirker R, Szczesna A, von Pawel J, Krzakowski M, Ramlau R, Park K et al. (2008). FLEX: a randomized, multicenter, phase III study of cetuximab in combination with cisplatin/vinorelbine (CV) versus CV alone in the first-line treatment of patients with advanced non-small cell lung cancer (NSCLC). J Clin Oncol 26 (Suppl): abstract 3.
Riely GJ, Politi KA, Miller VA, Pao W . (2006). Update on epidermal growth factor receptor mutations in non–small cell lung cancer. Clin Cancer Res 12: 7232–7241.
Rizvi N, Kris M, Miller V, Azzoli C, Krug L, Bekele S et al. (2007). A phase II study of XL647 in non-small cell lung cancer (NSCLC) patients enriched for presence of EGFR mutations: P3-136. J Thorac Oncol 2 (Suppl 4): S737.
Rosell R, Robinet G, Szczesna A, Ramlau R, Constenla M, Mennecier BC et al. (2008). Randomized phase II study of cetuximab plus cisplatin/vinorelbine compared with cisplatin/vinorelbine alone as first-line therapy in EGFR-expressing advanced non-small-cell lung cancer. Ann Oncol 19: 362–369.
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A et al. (2006). Paclitaxel-carboplatin alone or with bevcizumab for non-small-cell lung cancer. N Engl J Med 355: 2542–2550.
Scagliotti GV, De Marinis F, Rinaldi M, Crinò L, Gridelli C, Ricci S et al. (2002). Phase III randomized trial comparing three platinum-based doublets in advanced non–small-cell lung cancer. J Clin Oncol 20: 4285–4291.
Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C et al. (2008a). Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non–small-cell lung cancer. J Clin Oncol 26: 3543–3551.
Scagliotti G, von Pawel J, Reck M, Cupit L, Cihon F, DiMatteo S et al. (2008b). Sorafenib plus carboplatin/paclitaxel in chemonaive patients with stage IIIB-IV non-small cell lung cancer (NSCLC): interim analysis (IA) results from the phase III, randomized, double-blind, placebo-controlled, ESCAPE (Evaluation of Sorafenib, Carboplatin, and Paclitaxel Efficacy in NSCLC) Trial. J Thorac Oncol (European Lung Cancer Conference) 3 (4 Suppl 1): S9–S98; Late-breaking abstract 2750.
Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J et al. (2002). Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346: 92–98.
Sharma SV, Bell DW, Settleman J, Haber DA . (2007). Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 7: 169–181.
Shepherd FA, Rodrigues PJ, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S et al. (2005). Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353: 123–132.
Shih JY, Gow CH, Yang PC . (2005). EGFR mutation conferring primary resistance to gefitinib in non-small-cell lung cancer. N Engl J Med 353: 207–208.
Socinski MA, Novello S, Brahmer JR, Rosell R, Sanchez JM, Belani CP et al. (2008). Multicenter, phase II trial of sunitinib in previously treated, advanced non-small-cell lung cancer. J Clin Oncol 26: 650–656.
Stinchcombe TE, Socinski MA . (2008). Considerations for second-line therapy of non-small cell lung cancer. Oncologist 13 (Suppl 1): 28–36.
Tabernero J . (2007). The role of VEGF and EGFR inhibition: implications for combining anti-VEGF and anti-EGFR agents. Mol Cancer Res 5: 203–220.
Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J et al. (2005). Gefitinib plus BSC in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 366: 1527–1537.
Tsao M, Zhu C, Sakurada A, Chang I, Whitehead M, Kamei-Keid S et al. (2006). An analysis of the prognostic and predictive importance of K-ras mutation status in the National Cancer Institute of Canada Clinical Trials Group BR.21 study of erlotinib versus placebo in the treatment of non-small cell lung cancer. J Clin Oncol 24 (18S): (ASCO Annual Meeting Proceedings Part I), abstract 7005.
van Zandwijk N, Mathy A, Boerrigter L, Ruijter H, Tielen I, de Jong D et al. (2007). EGFR and KRAS mutations as criteria for treatment with tyrosine kinase inhibitors: retro- and prospective observations in non-small-cell lung cancer. Ann Oncol 18: 99–103.
Von Pawel J, Kaiser R, Eschbach C, Stefanie M, Love J, Gatzemeier U, Reck M . (2007). A double blind phase II study of BIBF 1120 in patients suffering from relapsed advanced non-small cell lung cancer (NSCLC). J Clin Oncol 25 (Suppl 18): abstract no. 7635 418s.
Wheatley-Price P, Ding K, Seymour L, Clark GM, Shepherd FA . (2008). Erlotinib for advanced non–small-cell lung cancer in the elderly: an analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol 26: 2350–2357.
Wissner A, Mansour TS . (2008). The development of HKI-272 and related compounds for the treatment of cancer. Arch Pharm (Weinheim) 341: 465–477.
Yang C, Shih J, Chao T, Tsai C, Su W, Hsia T et al. (2008). Use of BIBW 2992, a novel irreversible EGFR/HER2 TKI, to induce regression in patients with adenocarcinoma of the lung and activating EGFR mutations: preliminary results of a single-arm phase II clinical trial. J Clin Oncol 26: abstract 8026.
Ye D, Mendelsohn J, Fan Z . (1999). Augmentation of a humanized anti-HER2 mAb4D5 induced growth inhibition by a human-mouse chimeric anti-EGF receptor mAb C225. Oncogene 18: 731–738.
Acknowledgements
The author thanks Johnathan C Maher, PhD, of BlueSpark Healthcare for medical and editorial assistance in this paper. Financial support for medical and editorial assistance was provided by Boehringer Ingelheim Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burris, H. Shortcomings of current therapies for non-small-cell lung cancer: unmet medical needs. Oncogene 28 (Suppl 1), S4–S13 (2009). https://doi.org/10.1038/onc.2009.196
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2009.196