Abstract
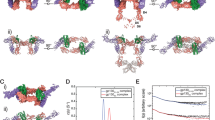
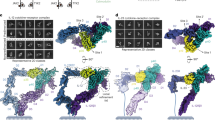
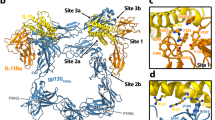
gp130 is a shared cytokine signaling receptor and the founding member of the 'tall' class of cytokine receptors. A crystal structure of the ligand-binding domains of gp130 in complex with human interleukin-6 (IL-6) and its a-receptor (IL-6Rα) revealed a hexameric architecture in which the gp130 membrane-distal regions were ∼100 Å apart, in contrast to the close apposition seen between short cytokine receptor complexes. Here we used single-particle EM to visualize the entire extracellular hexameric IL-6–IL-6Rα–gp130 complex, containing all six gp130 domains. The structure reveals that gp130 is bent such that the membrane-proximal domains of gp130 are close together at the cell surface, enabling activation of intracellular signaling. Variation in the receptor bend angles suggests a possible conformational transition from open to closed states upon ligand binding; this transition is probably representative of the other tall cytokine receptors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kishimoto, T., Akira, S., Narazaki, M. & Taga, T. Interleukin-6 family of cytokines and gp130. Blood 86, 1243–1254 (1995).
Bravo, J. & Heath, J.K. Receptor recognition by gp130 cytokines. EMBO J. 19, 2399–2411 (2000).
Kishimoto, T., Taga, T. & Akira, S. Cytokine signal transduction. Cell 76, 253–262 (1994).
Heinrich, P.C. et al. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 374, 1–20 (2003).
Bazan, J.F. Haemopoietic receptors and helical cytokines. Immunol. Today 11, 350–354 (1990).
Bravo, J., Staunton, D., Heath, J.K. & Jones, E.Y. Crystal structure of a cytokine-binding region of gp130. EMBO J. 17, 1665–1674 (1998).
Chow, D., He, X., Snow, A.L., Rose-John, S. & Garcia, K.C. Structure of an extracellular gp130 cytokine receptor signaling complex. Science 291, 2150–2155 (2001).
Boulanger, M.J. & Garcia, K.C. Shared cytokine signaling receptors: structural insights from the gp130 system. Adv. Protein Chem. 68, 107–146 (2004).
Boulanger, M.J., Chow, D.C., Brevnova, E.E. & Garcia, K.C. Hexameric structure and assembly of the interleukin-6/IL-6 α-receptor/gp130 complex. Science 300, 2101–2104 (2003).
Ihle, J.N. Cytokine receptor signalling. Nature 377, 591–594 (1995).
Wells, J.A. & de Vos, A.M. Hematopoietic receptor complexes. Annu. Rev. Biochem. 65, 609–634 (1996).
Kurth, I. et al. Importance of the membrane-proximal extracellular domains for activation of the signal transducer glycoprotein 130. J. Immunol. 164, 273–282 (2000).
Varghese, J.N. et al. Structure of the extracellular domains of the human interleukin-6 receptor α-chain. Proc. Natl. Acad. Sci. USA 99, 15959–15964 (2002).
Leahy, D.J., Erickson, H.P., Aukhil, I., Joshi, P. & Hendrickson, W.A. Crystallization of a fragment of human fibronectin: introduction of methionine by site-directed mutagenesis to allow phasing via selenomethionine. Proteins 19, 48–54 (1994).
Kossiakoff, A.A. & De Vos, A.M. Structural basis for cytokine hormone-receptor recognition and receptor activation. Adv. Protein Chem. 52, 67–108 (1998).
de Vos, A.M., Ultsch, M. & Kossiakoff, A.A. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science 255, 306–312 (1992).
Walter, M.R. et al. Crystal structure of a complex between interferon-gamma and its soluble high-affinity receptor. Nature 376, 230–235 (1995).
Somers, W., Ultsch, M., De Vos, A.M. & Kossiakoff, A.A. The X-ray structure of a growth hormone-prolactin receptor complex. Nature 372, 478–481 (1994).
Livnah, O. et al. Functional mimicry of a protein hormone by a peptide agonist: the EPO receptor complex at 2.8 Å. Science 273, 464–471 (1996).
Syed, R.S. et al. Efficiency of signalling through cytokine receptors depends critically on receptor orientation. Nature 395, 511–516 (1998).
Grotzinger, J., Kernebeck, T., Kallen, K.J. & Rose-John, S. IL-6 type cytokine receptor complexes: hexamer, tetramer or both? Biol. Chem. 380, 803–813 (1999).
Takagi, J., Petre, B.M., Walz, T. & Springer, T.A. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell 110, 599–611 (2002).
Pflanz, S., Kurth, I., Grotzinger, J., Heinrich, P.C. & Muller-Newen, G. Two different epitopes of the signal transducer gp130 sequentially cooperate on IL-6-induced receptor activation. J. Immunol. 165, 7042–7049 (2000).
Ohi, M., Li, Y., Cheng, Y. & Walz, T. Negative staining and image classification—powerful tools in modern electron microscopy. Biol. Proced. Online 6, 23–34 (2004).
Frank, J. et al. SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J. Struct. Biol. 116, 190–199 (1996).
Radermacher, M., Wagenknecht, T., Verschoor, A. & Frank, J. Three-dimensional reconstruction from a single-exposure, random conical tilt series applied to the 50S ribosomal subunit of Escherichia coli. J. Microsc. 146, 113–136 (1987).
Stewart, A. & Grigorieff, N. Noise bias in the refinement of structures derived from single particles. Ultramicroscopy 102, 67–84 (2004).
Rosenthal, P.B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 333, 721–745 (2003).
Jones, T.A., Zou, J.Y., Cowan, S.W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991).
Acknowledgements
We acknowledge D. Chow, N. Goriatcheva, M. Martick and L. Brevnova for assistance and helpful discussions, and Y. Cheng for support in data collection and image processing. We also acknowledge the Keck Foundation (K.C.G.), Pew Trust (K.C.G.) and US National Institutes of Health (NIH) AI51321 (K.C.G.) for support. The molecular EM facility at Harvard Medical School was established by a generous donation from the Giovanni Armenise Harvard Center for Structural Biology and is maintained by funds from NIH GM62580 (T.W.). G.S. is a Damon Runyon Fellow, supported by the Damon Runyon Cancer Research Foundation (DRG-#1824-04).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Class averages. (PDF 1254 kb)
Supplementary Fig. 2
Angular distribution plots and Fourier shell correlation curve. (PDF 307 kb)
Supplementary Fig. 3
Improvement of the density map for the IL-6–IL-6Rα–gp130 complex. (PDF 2739 kb)
Rights and permissions
About this article
Cite this article
Skiniotis, G., Boulanger, M., Garcia, K. et al. Signaling conformations of the tall cytokine receptor gp130 when in complex with IL-6 and IL-6 receptor. Nat Struct Mol Biol 12, 545–551 (2005). https://doi.org/10.1038/nsmb941
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb941
This article is cited by
-
Soluble receptors in cancer: mechanisms, clinical significance, and therapeutic strategies
Experimental & Molecular Medicine (2024)
-
The Human GP130 Cytokine Receptor and Its Expression—an Atlas and Functional Taxonomy of Genetic Variants
Journal of Clinical Immunology (2024)
-
Role of interleukin 6 and its soluble receptor on the diffusion barrier dysfunction of alveolar tissue
Biomedical Microdevices (2023)
-
β-Klotho promotes glycolysis and glucose-stimulated insulin secretion via GP130
Nature Metabolism (2022)
-
Autoinflammatory Pathogenesis and Targeted Therapy for Adult-Onset Still’s Disease
Clinical Reviews in Allergy & Immunology (2020)