Abstract
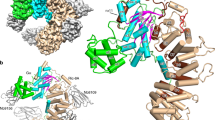
p115RhoGEF, a guanine nucleotide exchange factor (GEF) for Rho GTPase, is also a GTPase-activating protein (GAP) for G12 and G13 heterotrimeric Gα subunits. The GAP function of p115RhoGEF resides within the N-terminal region of p115RhoGEF (the rgRGS domain), which includes a module that is structurally similar to RGS (regulators of G-protein signaling) domains. We present here the crystal structure of the rgRGS domain of p115RhoGEF in complex with a chimera of Gα13 and Gαi1. Two distinct surfaces of rgRGS interact with Gα. The N-terminal βN–αN hairpin of rgRGS, rather than its RGS module, forms intimate contacts with the catalytic site of Gα. The interface between the RGS module of rgRGS and Gα is similar to that of a Gα–effector complex, suggesting a role for the rgRGS domain in the stimulation of the GEF activity of p115RhoGEF by Gα13.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kozasa, T. et al. p115 RhoGEF, a GTPase-activating protein for Gα12 and Gα13. Science 280, 2109–2111 (1998).
Hart, M.J. et al. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Gα13. Science 280, 2112–2114 (1998).
Sah, V.P., Seasholtz, T.M., Sagi, S.A. & Brown, J.H. The role of Rho in G protein–coupled receptor signal transduction. Annu. Rev. Pharmacol. Toxicol. 40, 459–489 (2000).
Buhl, A.M., Johnson, N.L., Dhanasekaran, N. & Johnson, G.L. Gα12 and Gα13 stimulate Rho-dependent stress fiber formation and focal adhesion assembly. J. Biol. Chem. 270, 24631–24634 (1995).
Gohla, A., Harhammer, R. & Schultz, G. The G-protein G13 but not G12 mediates signaling from lysophosphatidic acid receptor via epidermal growth factor receptor to Rho. J. Biol. Chem. 273, 4653–4659 (1998).
Cerione, R.A. & Zheng, Y. The Dbl family of oncogenes. Curr. Opin. Cell Biol. 8, 216–222 (1996).
Ross, E.M. & Wilkie, T.M. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu. Rev. Biochem. 69, 795–827 (2000).
Zheng, B., De Vries, L. & Gist Farquhar, M. Divergence of RGS proteins: evidence for the existence of six mammalian RGS subfamilies. Trends. Biochem. Sci. 24, 411–414 (1999).
Tesmer, J.J.G., Berman, D.M., Gilman, A.G. & Sprang, S.R. Structure of RGS4 bound to AIF4−-activated Giα1: stabilization of the transition state for GTP hydrolysis. Cell 89, 251–261 (1997).
Berman, D.M., Wilkie, T.M. & Gilman, A.G. GAIP and RGS4 are GTPase-activating proteins (GAPs) for the Gi subfamily of G protein α-subunits. Cell 86, 445–452 (1996).
Druey, K.M. & Kehrl, J.H. Inhibition of regulator of G protein signaling function by two mutant RGS4 proteins. Proc. Natl. Acad. Sci. USA 94, 12851–12856 (1997).
Srinivasa, S.P., Watson, N., Overton, M.C. & Blumer, K.J. Mechanism of RGS4, a GTPase-activating protein for G protein α-subunits. J. Biol. Chem. 273, 1529–1533 (1998).
Posner, B.A., Mukhopadhyay, S., Tesmer, J.J., Gilman, A.G. & Ross, E.M. Modulation of the affinity and selectivity of RGS protein interaction with G α-subunits by a conserved asparagine/serine residue. Biochemistry 38, 7773–7779 (1999).
Slep, K.C. et al. Structural determinants for regulation of phosphodiesterase by a G protein at 2.0 Å. Nature 409, 1071–1077 (2001).
Fukuhara, S., Chikumi, H. & Gutkind, J.S. Leukemia-associated Rho guanine nucleotide exchange factor (LARG) links heterotrimeric G proteins of the G (12) family to Rho. FEBS Lett. 485, 183–188 (2000).
Whitehead, I.P. et al. Expression cloning of lsc, a novel oncogene with structural similarities to the Dbl family of guanine nucleotide exchange factors. J. Biol. Chem. 271, 18643–18650 (1996).
Fukuhara, S., Murga, C., Zohar, M., Igishi, T. & Gutkind, J.S. A novel PDZ domain containing guanine nucleotide exchange factor links heterotrimeric G proteins to Rho. J. Biol. Chem. 274, 5868–5879 (1999).
Jackson, M. et al. Modulation of the neuronal glutamate transporter EAAT4 by two interacting proteins. Nature 410, 89–93 (2001).
Wells, C.D. et al. Mechanisms for reversible regulation between p115 RhoGEF and GTRAP48 and the G12 class of heterotrimeric G proteins. J. Biol. Chem. 277, 1174–1181 (2002).
Chen, Z., Singer, W.D., Wells, C.D., Sprang, S.R. & Sternweis, P.C. Mapping the Gα13 binding interface of the rgRGS domain of p115RhoGEF. J. Biol. Chem. 278, 9912–9919 (2003).
Chen, Z., Wells, C.D., Sternweis, P.C. & Sprang, S.R. Structure of the rgRGS domain of p115RhoGEF. Nat. Struct. Biol. 8, 805–809 (2001).
Longenecker, K.L., Lewis, M.E., Chikumi, H., Gutkind, J.S. & Derewenda, Z.S. Structure of the RGS-like domain from PDZ-RhoGEF: linking heterotrimeric G protein–coupled signaling to Rho GTPases. Structure 9, 559–569 (2001).
Singer, W.D., Miller, R.T. & Sternweis, P.C. Purification and characterization of the α-subunit of G13. J. Biol. Chem. 269, 19796–19802 (1994).
Wells, C.D., Gutowski, S., Bollag, G. & Sternweis, P.C. Identification of potential mechanisms for regulation of p115 RhoGEF through analysis of endogenous and mutant forms of the exchange factor. J. Biol. Chem. 276, 28897–28905 (2001).
Berlot, C.H. & Bourne, H.R. Identification of effector-activating residues of Gs α. Cell 68, 911–922 (1992).
Skiba, N.P., Bae, H. & Hamm, H.E. Mapping of effector binding sites of transducin α-subunit using Gαt/Gαi1 chimeras. J. Biol. Chem. 271, 413–424 (1996).
Eisenhaure, T.M., Francis, S.A., Willison, L.D., Coughlin, S.R. & Lerner, D.J. The Rho guanine nucleotide exchange factor Lsc homo-oligomerizes and is negatively regulated through domains in its carboxyl terminus that are absent in novel splenic isoforms. J. Biol. Chem. 278, 30975–30984 (2003).
Chikumi, H. et al. Homo- and hetero-oligomerization of PDZ-RhoGEF, LARG and p115RhoGEF by their C-terminal region regulates their in vivo Rho GEF activity and transforming potential. Oncogene 23, 233–240 (2004).
Coleman, D.E. et al. Structures of active conformations of Giα1 and the mechanism of GTP hydrolysis. Science 265, 1405–1412 (1994).
Sondek, J., Lambright, D.G., Noel, J.P., Hamm, H.E. & Sigler, P.B. GTPase mechanism of G proteins from the 1.7 Å crystal structure of transducin α-GDP-AIF4−. Nature 372, 276–279 (1994).
Tesmer, J.J., Sunahara, R.K., Gilman, A.G. & Sprang, S.R. Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gsα.GTPγS. Science 278, 1907–1916 (1997).
Sterne-Marr, R. et al. G protein–coupled receptor kinase 2/Gαq/11 interaction. A novel surface on a regulator of G protein signaling homology domain for binding Gα subunits. J. Biol. Chem. 278, 6050–6058 (2003).
Horton, R.M., Hunt, H.D., Ho, S.N., Pullen, J.K. & Pease, L.R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77, 61–68 (1989).
Lee, E., Linder, M.E. & Gilman, A.G. Expression of G protein α-subunits in Escherichia coli. Methods Enzymol. 237, 146–164 (1994).
Wells, C., Jiang, X., Gutowski, S. & Sternweis, P.C. Functional characterization of p115 RhoGEF. Methods Enzymol. 345, 371–382 (2002).
Northup, J.K., Smigel, M.D. & Gilman, A.G. The guanine nucleotide activating site of the regulatory component of adenylate cyclase. Identification by ligand binding. J. Biol. Chem. 257, 11416–11423 (1982).
Adhikari, A. & Sprang, S.R. Thermodynamic characterization of the binding of activator of G protein signaling 3 (AGS3) and peptides derived from AGS3 with Gαi1. J. Biol. Chem. 278, 51825–51832 (2003).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Navaza, J. AMoRe: an automated package for molecular replacement. Acta Crystallogr. A 50, 157–163 (1994).
Jones, T.A., Zou, J.Y., Cowan, S.W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991).
Brunger, A.T. et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998).
Laskowski, R.A., MacArthur, M.W., Moss, D.S. & Thornton, J.M. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26, 283–291 (1993).
Ramachandran, G.N. & Sassiekharan, V. Conformation of polypeptides and proteins. Adv. Protein Chem. 28, 283–437 (1968).
Kraulis, P.J. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24, 946–950 (1991).
Guex, N. & Peitsch, M.C. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18, 2714–2723 (1997).
Read, R.J. Improved Fourier coefficients for maps using phases from partial structures with errors. Acta Crystallogr. A 42, 140–149 (1986).
Acknowledgements
We thank W. Chen, S. Gutowski, J. Hadas and A. Badejo for technical assistance, and the staff of Advanced Light Source for assistance with data collection. This work was supported by US National Institutes of Health Grants GM31954 (to P.C.S.), and DK46371 (to S.R.S.), by the Robert A. Welch foundation (to P.C.S. and S.R.S.), the Alfred and Mabel Gilman Chair in Molecular Pharmacology (P.C.S.) and the John W. and Rhonda K. Pate Professorship in Biochemistry (to S.R.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Structural alignment of helical domains of Gα13 and Gαi1. (PDF 526 kb)
Supplementary Fig. 2
The βN–αN segment of rgRGS fails to stimulate the GTPase activity of Gαi1. (PDF 291 kb)
Supplementary Fig. 3
Changes in α3–β5 region of Gα13/i-5 do not make it a better substrate for rgRGS. (PDF 418 kb)
Supplementary Table 1
Basic residues in the helical domain and switch regions unique to the G12 class Gα subunits. (PDF 17 kb)
Supplementary Table 2
List of protein-protein contacts between the rgRGS domain and Gα13/i-5. (PDF 526 kb)
Rights and permissions
About this article
Cite this article
Chen, Z., Singer, W., Sternweis, P. et al. Structure of the p115RhoGEF rgRGS domain–Gα13/i1 chimera complex suggests convergent evolution of a GTPase activator. Nat Struct Mol Biol 12, 191–197 (2005). https://doi.org/10.1038/nsmb888
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb888
This article is cited by
-
Convergent selective signaling impairment exposes the pathogenicity of latrophilin-3 missense variants linked to inheritable ADHD susceptibility
Molecular Psychiatry (2022)
-
Interaction kinetics between p115-RhoGEF and Gα13 are determined by unique molecular interactions affecting agonist sensitivity
Communications Biology (2022)
-
Gα12/13 signaling in metabolic diseases
Experimental & Molecular Medicine (2020)
-
Efficacy of a novel water-soluble curcumin derivative versus sildenafil citrate in mediating erectile function
International Journal of Impotence Research (2015)
-
Determinants at the N- and C-termini of Gα12 required for activation of Rho-mediated signaling
Journal of Molecular Signaling (2013)