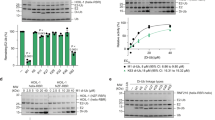
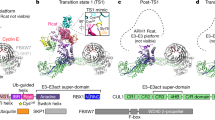
Abstract
Post-translational modification with the ubiquitin-related protein SUMO1 requires the E1 enzyme Aos1–Uba2 and the E2 enzyme Ubc9. Distinct E3 ligases strongly enhance modification of specific targets. The SUMO E3 ligase RanBP2 (also known as Nup358) has no obvious similarity to RING- or HECT-type enzymes. Here we show that RanBP2's 30-kDa catalytic fragment is a largely unstructured protein. Despite two distinct but partially overlapping 79-residue catalytic domains, one of which is sufficient for maximal activity, RanBP2 binds to Ubc9 in a 1:1 stoichiometry. The identification of nine RanBP2 and three Ubc9 side chains that are important for RanBP2-dependent SUMOylation indicates largely hydrophobic interactions. These properties distinguish RanBP2 from all other known E3 ligases, and we speculate that RanBP2 exerts its catalytic effect by altering Ubc9's properties rather than by mediating target interactions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Melchior, F. SUMO—nonclassical ubiquitin. Annu. Rev. Cell Dev. Biol. 16, 591–626 (2000).
Müller, S., Hoege, C., Pyrowolakis, G. & Jentsch, S. SUMO, ubiquitin's mysterious cousin. Nat. Rev. Mol. Cell Biol. 2, 202–210 (2001).
Kim, K.I., Baek, S.H. & Chung, C.H. Versatile protein tag, SUMO: its enzymology and biological function. J. Cell Physiol. 191, 257–268 (2002).
Verger, A., Perdomo, J. & Crossley, M. Modification with SUMO. EMBO Rep. 4, 137–142 (2003).
Melchior, F., Schergaut, M. & Pichler, A. SUMO: ligases, isopeptidases and nuclear pores. Trends Biochem. Sci. 28, 612–618 (2003).
Hershko, A. & Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem. 67, 425–479 (1998).
Pickart, C.M. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 70, 503–533 (2001).
Jentsch, S. & Pyrowolakis, G. Ubiquitin and its kin: how close are the family ties? Trends Cell Biol. 10, 335–342 (2000).
Schwarz, D.C. & Hochstrasser, M. A superfamily of protein tags: ubiquitin, SUMO and related modifiers. Trends Biochem. Sci. 28, 321–328 (2003).
Weissman, A.M. Themes and variations on ubiquitylation. Nat. Rev. Mol. Cell Biol. 2, 169–178 (2001).
Cyr, D.M., Hohfeld, J. & Patterson, C. Protein quality control: U-box-containing E3 ubiquitin ligases join the fold. Trends Biochem. Sci. 27, 368–375 (2002).
Fang, S., Lorick, K.L., Jensen, J.P. & Weissman, A.M. RING finger ubiquitin protein ligases: implications for tumorigenesis, metastasis and for molecular targets in cancer. Semin. Cancer Biol. 13, 5–14 (2003).
Hatakeyama, S. & Nakayama, K.I. U-box proteins as a new family of ubiquitin ligases. Biochem. Biophys. Res. Commun. 302, 635–645 (2003).
Deshaies, R.J. SCF and Cullin/Ring H2-based ubiquitin ligases. Annu. Rev. Cell Dev. Biol. 15, 435–467 (1999).
Peters, J.M. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol. Cell 9, 931–943 (2002).
Schmidt, D. & Muller, S. PIAS/SUMO: new partners in transcriptional regulation. Cell. Mol. Life Sci. 60, 2561–2574 (2003).
Kagey, M.H., Melhuish, T.A. & Wotton, D. The polycomb protein Pc2 is a SUMO E3. Cell 113, 127–137 (2003).
Pichler, A., Gast, A., Seeler, J.S., Dejean, A. & Melchior, F. The nucleoporin RanBP2 has SUMO1 E3 ligase activity. Cell 108, 109–120 (2002).
Kirsh, O. et al. The SUMO E3 ligase RanBP2 promotes modification of the HDAC4 deacetylase. EMBO J. 21, 2682–2691 (2002).
Wu, J., Matunis, M.J., Kraemer, D., Blobel, G. & Coutavas, E. Nup358, a cytoplasmically exposed nucleoporin with peptide repeats, Ran-GTP binding sites, zinc fingers, a cyclophilin A homologous domain, and a leucine-rich region. J. Biol. Chem. 270, 14209–14213 (1995).
Yokoyama, N. et al. A giant nucleopore protein that binds Ran/TC4. Nature 376, 184–188 (1995).
Mahajan, R., Delphin, C., Guan, T., Gerace, L. & Melchior, F. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell 88, 97–107 (1997).
Matunis, M.J., Wu, J. & Blobel, G. SUMO-1 modification and its role in targeting the Ran GTPase-activating protein, RanGAP1, to the nuclear pore complex. J. Cell Biol. 140, 499–509 (1998).
Saitoh, H., Pu, R., Cavenagh, M. & Dasso, M. RanBP2 associates with Ubc9p and a modified form of RanGAP1. Proc. Natl. Acad. Sci. USA 94, 3736–3741 (1997).
Saitoh, H., Pizzi, M.D. & Wang, J. Perturbation of SUMOlation enzyme Ubc9 by distinct domain within nucleoporin RanBP2/Nup358. J. Biol. Chem. 277, 4755–4763 (2002).
Kahyo, T., Nishida, T. & Yasuda, H. Involvement of PIAS1 in the SUMOylation of tumor suppressor p53. Mol. Cell 8, 713–718 (2001).
Schmidt, D. & Muller, S. Members of the PIAS family act as SUMO ligases for c-Jun and p53 and repress p53 activity. Proc. Natl. Acad. Sci. USA 99, 2872–2877 (2002).
Sachdev, S. et al. PIASy, a nuclear matrix-associated SUMO E3 ligase, represses LEF1 activity by sequestration into nuclear bodies. Genes Dev. 15, 3088–3103 (2001).
Wu, P.Y. et al. A conserved catalytic residue in the ubiquitin-conjugating enzyme family. EMBO J. 22, 5241–5250 (2003).
Wyatt, P.J. Light scattering and the absolute characterization of macromolecules. Anal. Chim. Acta 272, 1–40 (1993).
Bernier-Villamor, V., Sampson, D.A., Matunis, M.J. & Lima, C.D. Structural basis for E2-mediated SUMO conjugation revealed by a complex between ubiquitin-conjugating enzyme Ubc9 and RanGAP1. Cell 108, 345–356 (2002).
Gmachl, M., Gieffers, C., Podtelejnikov, A.V., Mann, M. & Peters, J.M. The RING-H2 finger protein APC11 and the E2 enzyme UBC4 are sufficient to ubiquitinate substrates of the anaphase-promoting complex. Proc. Natl. Acad. Sci. USA 97, 8973–8978 (2000).
Leverson, J.D. et al. The APC11 RING-H2 finger mediates E2-dependent ubiquitination. Mol. Biol. Cell 11, 2315–2325 (2000).
Tang, Z. et al. APC2 Cullin protein and APC11 RING protein comprise the minimal ubiquitin ligase module of the anaphase-promoting complex. Mol. Biol. Cell 12, 3839–3851 (2001).
Seeler, J.S. et al. Common properties of nuclear body protein SP100 and TIF1α chromatin factor: role of SUMO modification. Mol. Cell. Biol. 21, 3314–3324 (2001).
Sapetschnig, A. et al. Transcription factor Sp3 is silenced through SUMO modification by PIAS1. EMBO J. 21, 5206–5215 (2002).
Tong, H., Hateboer, G., Perrakis, A., Bernards, R. & Sixma, T.K. Crystal structure of murine/human Ubc9 provides insight into the variability of the ubiquitin-conjugating system. J. Biol. Chem. 272, 21381–21387 (1997).
Acknowledgements
Our special thanks go to A. Klanner, J. Vordemann, P. van Dijk and E. Stieger for excellent technical assistance, to members of the lab and L. Hengst for sharing reagents and advice. We acknowledge L. Moroder and E. Weyher (Max Planck Institute for Biochemistry) for CD spectroscopy, and J. Velgaard Olsen (University of Southern Denmark) for mass spectrometry analysis. We thank J. Seeler, A. Dejean, R. Grosschedl, G. Suske, M. Oren and C. Lima for providing clones for GST-Sp100, GST-HDAC4, His-Lef-1, GST-PIAS1, GST-p53, and His-Ubc9, respectively. Funding was provided by BioFUTURE grant 0311869 to F.M.; P.K. was funded through grant NWO-MW901-02-223.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
N85Q Ubc9 in thioester bond formation and sumoylation. (PDF 72 kb)
Supplementary Fig. 2
Molecular mass of the RanBP2–Ubc9 complex. (PDF 57 kb)
Supplementary Fig. 3
Activity and binding for different RanBP2 fragments. (PDF 40 kb)
Supplementary Fig. 4
Sequence alignment of RanBP2's E3 ligase domain. (PDF 46 kb)
Rights and permissions
About this article
Cite this article
Pichler, A., Knipscheer, P., Saitoh, H. et al. The RanBP2 SUMO E3 ligase is neither HECT- nor RING-type. Nat Struct Mol Biol 11, 984–991 (2004). https://doi.org/10.1038/nsmb834
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb834
This article is cited by
-
SUMOylation targeting mitophagy in cardiovascular diseases
Journal of Molecular Medicine (2022)
-
E2 enzymes: more than just middle men
Cell Research (2016)
-
Regulation of aPKC activity by Nup358 dependent SUMO modification
Scientific Reports (2016)
-
The RanBP2/RanGAP1*SUMO1/Ubc9 SUMO E3 ligase is a disassembly machine for Crm1-dependent nuclear export complexes
Nature Communications (2016)
-
A new vertebrate SUMO enzyme family reveals insights into SUMO-chain assembly
Nature Structural & Molecular Biology (2015)