Abstract
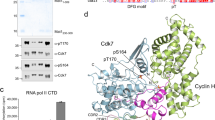
Cdk7 performs two essential but distinct functions as a CDK-activating kinase (CAK) required for cell-cycle progression and as the RNA polymerase II (Pol II) CTD kinase of general transcription factor IIH. To investigate the substrate specificity underlying this dual function, we created an analog-sensitive (AS) Cdk7 able to use bulky ATP derivatives. Cdk7-AS–cyclin H–Mat1 phosphorylates ∼10–15 endogenous polypeptides in nuclear extracts. We identify seven of these as known and previously unknown Cdk7 substrates that define two classes: proteins such as Pol II and transcription elongation factor Spt5, recognized efficiently only by the fully activated Cdk7 complex, through sequences surrounding the site of phosphorylation; and CDKs, targeted equivalently by all active forms of Cdk7, dependent on substrate motifs remote from the phosphoacceptor residue. Thus, Cdk7 accomplishes dual functions in cell-cycle control and transcription not through promiscuity but through distinct, stringent modes of substrate recognition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Fesquet, D. et al. The MO15 gene encodes the catalytic subunit of a protein kinase that activates cdc2 and other cyclin-dependent kinases (CDKs) through phosphorylation of Thr161 and its homologues. EMBO J. 12, 3111–3121 (1993).
Poon, R.Y., Yamashita, K., Adamczewski, J.P., Hunt, T. & Shuttleworth, J. The cdc2-related protein p40MO15 is the catalytic subunit of a protein kinase that can activate p33cdk2 and p34cdc2. EMBO J. 12, 3123–3132 (1993).
Solomon, M.J., Harper, J.W. & Shuttleworth, J. CAK, the p34cdc2 activating kinase, contains a protein identical or closely related to p40MO15. EMBO J. 12, 3133–3142 (1993).
Fisher, R.P. & Morgan, D.O. A novel cyclin associates with MO15/CDK7 to form the CDK-activating kinase. Cell 78, 713–724 (1994).
Mäkelä, T.P. et al. A cyclin associated with the CDK-activating kinase MO15. Nature 371, 254–257 (1994).
Devault, A. et al. MAT1 ('ménage à trois') a new RING finger protein subunit stabilizing cyclin H-cdk7 complexes in starfish and Xenopus CAK. EMBO J. 14, 5027–5036 (1995).
Fisher, R.P., Jin, P., Chamberlin, H.M. & Morgan, D.O. Alternative mechanisms of CAK assembly require an assembly factor or an activating kinase. Cell 83, 47–57 (1995).
Tassan, J.-P. et al. In vitro assembly of a functional human cdk7/cyclin H complex requires MAT1, a novel 36 kD RING finger protein. EMBO J. 14, 5608–5617 (1995).
Roy, R. et al. The MO15 cell cycle kinase is associated with the TFIIH transcription-DNA repair factor. Cell 79, 1093–1101 (1994).
Serizawa, H. et al. Association of Cdk-activating kinase subunits with transcription factor TFIIH. Nature 374, 280–282 (1995).
Shiekhattar, R. et al. Cdk-activating kinase (CAK) complex is a component of human transcription factor IIH. Nature 374, 283–287 (1995).
Harper, J.W. & Elledge, S.J. The role of Cdk7 in CAK function, a retro-retrospective. Genes Dev. 12, 285–289 (1998).
Larochelle, S., Pandur, J., Fisher, R.P., Salz, H.K. & Suter, B. Cdk7 is essential for mitosis and for in vivo Cdk-activating kinase activity. Genes Dev. 12, 370–381 (1998).
Hermand, D. et al. Fission yeast Csk1 is a CAK-activating kinase (CAKAK). EMBO J. 17, 7230–7238 (1998).
Lee, K.M., Saiz, J.E., Barton, W.A. & Fisher, R.P. Cdc2 activation in fission yeast depends on Mcs6 and Csk1, two partially redundant Cdk-activating kinases CAKs). Curr. Biol. 9, 441–444 (1999).
Saiz, J.E. & Fisher, R.P. A CDK-activating kinase network is required in cell cycle control and transcription in fission yeast. Curr. Biol. 12, 1100–1105 (2002).
Wallenfang, M.R. & Seydoux, G. cdk-7 is required for mRNA transcription and cell cycle progression in Caenorhabditis elegans embryos. Proc. Natl. Acad. Sci. USA 99, 5527–5532 (2002).
Poon, R.Y. & Hunter, T. Innocent bystanders or chosen collaborators? Curr. Biol. 5, 1243–1247 (1995).
Garrett, S. et al. Reciprocal activation by cyclin-dependent kinases 2 and 7 is directed by substrate specificity determinants outside the T-loop. Mol. Cell. Biol. 21, 88–99 (2001).
Trigon, S. et al. Characterization of the residues phosphorylated in vitro by different C-terminal domain kinases. J. Biol. Chem. 273, 6769–6775 (1998).
Ramanathan, Y. et al. Three RNA polymerase II carboxyl-terminal domain kinases display distinct substrate preferences. J. Biol. Chem. 276, 10913–10920 (2001).
Ko, L.J. et al. p53 is phosphorylated by CDK7-cyclin H in a p36MAT1-dependent manner. Mol. Cell. Biol. 17, 7220–7229 (1997).
Rochette-Egly, C., Adam, S., Rossignol, M., Egly, J.-M. & Chambon, P. Stimulation of RARα activation function AF-1 through binding to the general transcription factor TFIIH and phosphorylation by CDK7. Cell 90, 97–107 (1997).
Vandel, L. & Kouzarides, T. Residues phosphorylated by TFIIH are required for E2F–1 degradation during S-phase. EMBO J. 18, 4280–4291 (1999).
Inamoto, S., Segil, N., Pan, Z.-Q., Kimura, M. & Roeder, R.G. The cyclin-dependent kinase-activating kinase (CAK) assembly factor, MAT1, targets and enhances CAK activity on the POU domains of octamer transcription factors. J. Biol. Chem. 272, 29852–29858 (1997).
Aprelikova, O., Xiong, Y. & Liu, E.T. Both p16 and p21 families of cyclin-dependent kinase (CDK) inhibitors block the phosphorylation of cyclin-dependent kinases by the CDK-activating kinase. J. Biol. Chem. 270, 18195–18197 (1995).
Ubersax, J.A. et al. Targets of the cyclin-dependent kinase Cdk1. Nature 425, 859–864 (2003).
Nash, P. et al. Multisite phosphorylation of a CDK inhibitor sets a threshold for the onset of DNA replication. Nature 414, 514–521 (2001).
Rossignol, M., Kolb-Cheynel, I. & Egly, J.-M. Substrate specificity of the cdk-activating kinase (CAK) is altered upon association with TFIIH. EMBO J. 16, 1628–1637 (1997).
Yankulov, K.Y. & Bentley, D.L. Regulation of CDK7 substrate specificity by MAT1 and TFIIH. EMBO J. 16, 1638–1646 (1997).
Larochelle, S. et al. T-loop phosphorylation stabilizes the CDK7-cyclin H-MAT1 complex in vivo and regulates its CTD kinase activity. EMBO J. 20, 3749–3759 (2001).
Shah, K., Liu, Y., Deirmengian, C. & Shokat, K.M. Engineering unnatural nucleotide specificity for Rous sarcoma virus tyrosine kinase to uniquely label its direct substrates. Proc. Natl. Acad. Sci. USA 94, 3565–3570 (1997).
Kraybill, B.C., Elkin, L.L., Blethrow, J.D., Morgan, D.O. & Shokat, K.M. Inhibitor scaffolds as new allele specific kinase substrates. J. Am. Chem. Soc. 124, 12118–12128 (2002).
Cornelis, S. et al. Identification and characterization of a novel cell cycle-regulated internal ribosome entry site. Mol. Cell 5, 597–605 (2000).
Wada, T. et al. DSIF, a novel transcription elongation factor that regulates RNA polymerase II processivity, is composed of human Spt4 and Spt5 homologs. Genes Dev. 12, 343–356 (1998).
Wada, T., Takagi, T., Yamaguchi, Y., Watanabe, D. & Handa, H. Evidence that P-TEFb alleviates the negative effect of DSIF on RNA polymerase II-dependent transcription in vitro. EMBO J. 17, 7395–7403 (1998).
Ivanov, D., Kwak, Y.T., Guo, J. & Gaynor, R.B. Domains in the SPT5 protein that modulate its transcriptional regulatory properties. Mol. Cell. Biol. 20, 2970–2983 (2000).
Russo, A.A., Jeffrey, P.D. & Pavletich, N.P. Structural basis of cyclin-dependent kinase activation by phosphorylation. Nat. Struct. Biol. 3, 696–700 (1996).
Specht, K.M. & Shokat, K.M. The emerging power of chemical genetics. Curr. Opin. Cell Biol. 14, 155–159 (2002).
Poon, R.Y. & Hunter, T. Dephosphorylation of Cdk2 Thr160 by the cyclin-dependent kinase-interacting phosphatase KAP in the absence of cyclin. Science 270, 90–93 (1995).
Cheng, A., Ross, K.E., Kaldis, P. & Solomon, M.J. Dephosphorylation of cyclin-dependent kinases by type 2C protein phosphatases. Genes Dev. 13, 2946–2957 (1999).
Chen, J., Larochelle, S., Li, X. & Suter, B. Xpd/Ercc2 regulates CAK activity and mitotic progression. Nature 424, 228–232 (2003).
Habelhah, H. et al. Identification of new JNK substrate using ATP pocket mutant JNK and a corresponding ATP analogue. J. Biol. Chem. 276, 18090–18095 (2001).
Liu, Y. et al. Two cyclin-dependent kinases promote RNA polymerase II transcription and formation of the scaffold complex. Mol. Cell. Biol. 24, 1721–1735 (2004).
Rickert, P., Corden, J.L. & Lees, E. Cyclin C/CDK8 and cyclin H/CDK7/p36 are biochemically distinct CTD kinases. Oncogene 18, 1093–1102 (1999).
Pinhero, R., Liaw, P., Bertens, K. & Yankulov, K. Three cyclin-dependent kinases preferentially phosphorylate different parts of the C-terminal domain of the large subunit of RNA polymerase II. Eur. J. Biochem. 271, 1004–1014 (2004).
Hu, D., Mayeda, A., Trembley, J.H., Lahti, J.M. & Kidd, V.J. CDK11 complexes promote pre-mRNA splicing. J. Biol. Chem. 278, 8623–8629 (2003).
Li, T., Inoue, A., Lahti, J.M. & Kidd, V.J. Failure to proliferate and mitotic arrest of CDK11(p110/p58)-null mutant mice at the blastocyst stage of embryonic cell development. Mol. Cell. Biol. 24, 3188–3197 (2004).
Wen, Y. & Shatkin, A.J. Transcription elongation factor hSPT5 stimulates mRNA capping. Genes Dev. 13, 1774–1779 (1999).
Pei, Y. & Shuman, S. Interactions between fission yeast mRNA capping enzymes and elongation factor Spt5. J. Biol. Chem. 277, 19639–19648 (2002).
Orphanides, G. & Reinberg, D. A unified theory of gene expression. Cell 108, 439–451 (2002).
Fisher, R.P. Reconstitution of mammalian CDK-activating kinase. Methods Enzymol. 283, 256–270 (1997).
Dignam, J.D., Lebovitz, R.M. & Roeder, R.G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11, 1475–1489 (1983).
Gamble, M.J., Erdjument-Bromage, H., Tempst, P., Freedman, L.P. & Fisher, R.P. The histone chaperone TAF-I/SET/INHAT is required for transcription in vitro of chromatin templates. Mol. Cell. Biol. 25, 797–807 (2005).
Acknowledgements
We thank D.O. Morgan (University of California at San Francisco) for providing vectors for production of recombinant NDPK and for critical review of the manuscript; M.B. Mathews (University of Medicine and Dentistry of New Jersey) and D.H. Price (University of Iowa) for providing human cyclin T1 cDNAs; Y. Ramanathan for construction of human Cdk9 and cyclin T1 expression vectors; J. Singer for the purification of Csk1; G. Livshits for cloning Cdk11; H. Erdjument-Bromage and P. Tempst for MS identification of proteins; and A. Koff for critical review of the manuscript. HeLa cells were grown by the US National Cell Culture Center. This work was supported by a research fellowship award to S.L. from the National Cancer Institute of Canada, supported with funds provided by the Terry Fox run and by US National Institutes of Health grants GM56985 and DK45460 to R.P.F and EB001987 to K.M.S.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Structures of 1-NMPP1, N6-(benzyl)-ATP and 3-(benzyl)-PPTP (PDF 123 kb)
Supplementary Fig. 2
Effect of ATP on specificity of labeling. (PDF 593 kb)
Supplementary Fig. 3
Immunoprecipitation of labeled Cdks. (PDF 246 kb)
Supplementary Fig. 4
Initial purification steps for Cdk7-AS substrates. (PDF 326 kb)
Supplementary Table 1
Nucleotide specificity of Cdk7-AS. (PDF 27 kb)
Rights and permissions
About this article
Cite this article
Larochelle, S., Batliner, J., Gamble, M. et al. Dichotomous but stringent substrate selection by the dual-function Cdk7 complex revealed by chemical genetics. Nat Struct Mol Biol 13, 55–62 (2006). https://doi.org/10.1038/nsmb1028
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb1028
This article is cited by
-
Multi-omics investigation reveals functional specialization of transcriptional cyclin dependent kinases in cancer biology
Scientific Reports (2022)
-
Distinct Cdk9-phosphatase switches act at the beginning and end of elongation by RNA polymerase II
Nature Communications (2020)
-
A computational protocol to evaluate the effects of protein mutants in the kinase gatekeeper position on the binding of ATP substrate analogues
BMC Research Notes (2017)
-
JNKs function as CDK4-activating kinases by phosphorylating CDK4 and p21
Oncogene (2017)
-
Identification of lipocalin-2 as a PKCδ phosphorylation substrate in neutrophils
Journal of Biomedical Science (2015)