Abstract
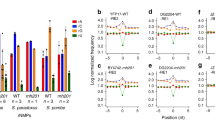
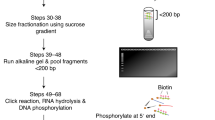
Ribonucleotides are frequently incorporated into DNA during replication in eukaryotes. Here we map genome-wide distribution of these ribonucleotides as markers of replication enzymology in budding yeast, using a new 5′ DNA end–mapping method, hydrolytic end sequencing (HydEn-seq). HydEn-seq of DNA from ribonucleotide excision repair–deficient strains reveals replicase- and strand-specific patterns of ribonucleotides in the nuclear genome. These patterns support the roles of DNA polymerases α and δ in lagging-strand replication and of DNA polymerase ɛ in leading-strand replication. They identify replication origins, termination zones and variations in ribonucleotide incorporation frequency across the genome that exceed three orders of magnitude. HydEn-seq also reveals strand-specific 5′ DNA ends at mitochondrial replication origins, thus suggesting unidirectional replication of a circular genome. Given the conservation of enzymes that incorporate and process ribonucleotides in DNA, HydEn-seq can be used to track replication enzymology in other organisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Johansson, E. & Dixon, N. Replicative DNA polymerases. Cold Spring Harb. Perspect. Biol. 5, a012799 (2013).
Georgescu, R.E. et al. Mechanism of asymmetric polymerase assembly at the eukaryotic replication fork. Nat. Struct. Mol. Biol. 21, 664–670 (2014).
Burgers, P.M. Polymerase dynamics at the eukaryotic DNA replication fork. J. Biol. Chem. 284, 4041–4045 (2009).
Pursell, Z.F., Isoz, I., Lundstrom, E.B., Johansson, E. & Kunkel, T.A. Yeast DNA polymerase ɛ participates in leading-strand DNA replication. Science 317, 127–130 (2007).
Lujan, S.A. et al. Mismatch repair balances leading and lagging strand DNA replication fidelity. PLoS Genet. 8, e1003016 (2012).
Lujan, S.A. et al. Heterogeneous polymerase fidelity and mismatch repair bias genome variation and composition. Genome Res. 24, 1751–1764 (2014).
Nick McElhinny, S.A., Gordenin, D.A., Stith, C.M., Burgers, P.M. & Kunkel, T.A. Division of labor at the eukaryotic replication fork. Mol. Cell 30, 137–144 (2008).
Larrea, A.A. et al. Genome-wide model for the normal eukaryotic DNA replication fork. Proc. Natl. Acad. Sci. USA 107, 17674–17679 (2010).
Balakrishnan, L. & Bambara, R.A. Okazaki fragment metabolism. Cold Spring Harb. Perspect. Biol. 5, a010173 (2013).
Smith, D.J. & Whitehouse, I. Intrinsic coupling of lagging-strand synthesis to chromatin assembly. Nature 483, 434–438 (2012).
McGuffee, S.R., Smith, D.J. & Whitehouse, I. Quantitative, genome-wide analysis of eukaryotic replication initiation and termination. Mol. Cell 50, 123–135 (2013).
Yeeles, J.T., Poli, J., Marians, K.J. & Pasero, P. Rescuing stalled or damaged replication forks. Cold Spring Harb. Perspect. Biol. 5, a012815 (2013).
Ghosal, G. & Chen, J. DNA damage tolerance: a double-edged sword guarding the genome. Transl. Cancer Res. 2, 107–129 (2013).
Wanrooij, S. & Falkenberg, M. The human mitochondrial replication fork in health and disease. Biochim. Biophys. Acta 1797, 1378–1388 (2010).
Gerhold, J.M., Aun, A., Sedman, T., Joers, P. & Sedman, J. Strand invasion structures in the inverted repeat of Candida albicans mitochondrial DNA reveal a role for homologous recombination in replication. Mol. Cell 39, 851–861 (2010).
Reyes, A. et al. Mitochondrial DNA replication proceeds via a 'bootlace' mechanism involving the incorporation of processed transcripts. Nucleic Acids Res. 41, 5837–5850 (2013).
Nick McElhinny, S.A. et al. Genome instability due to ribonucleotide incorporation into DNA. Nat. Chem. Biol. 6, 774–781 (2010).
Sparks, J.L. et al. RNase H2-initiated ribonucleotide excision repair. Mol. Cell 47, 980–986 (2012).
Williams, J.S. & Kunkel, T.A. Ribonucleotides in DNA: origins, repair and consequences. DNA Repair (Amst.) 19, 27–37 (2014).
Williams, J.S. et al. Topoisomerase 1-mediated removal of ribonucleotides from nascent leading-strand DNA. Mol. Cell 49, 1010–1015 (2013).
Lujan, S.A., Williams, J.S., Clausen, A.R., Clark, A.B. & Kunkel, T.A. Ribonucleotides are signals for mismatch repair of leading-strand replication errors. Mol. Cell 50, 437–443 (2013).
Pavlov, Y.I., Shcherbakova, P.V. & Kunkel, T.A. In vivo consequences of putative active site mutations in yeast DNA polymerases α, ɛ, δ, and ζ. Genetics 159, 47–64 (2001).
Nick McElhinny, S.A. et al. Abundant ribonucleotide incorporation into DNA by yeast replicative polymerases. Proc. Natl. Acad. Sci. USA 107, 4949–4954 (2010).
Siow, C.C., Nieduszynska, S.R., Muller, C.A. & Nieduszynski, C.A. OriDB, the DNA replication origin database updated and extended. Nucleic Acids Res. 40, D682–D686 (2012).
Niimi, A. et al. Palm mutants in DNA polymerases α and η alter DNA replication fidelity and translesion activity. Mol. Cell. Biol. 24, 2734–2746 (2004).
Nick McElhinny, S.A., Stith, C.M., Burgers, P.M. & Kunkel, T.A. Inefficient proofreading and biased error rates during inaccurate DNA synthesis by a mutant derivative of Saccharomyces cerevisiae DNA polymerase δ. J. Biol. Chem. 282, 2324–2332 (2007).
Nick McElhinny, S.A., Kissling, G.E. & Kunkel, T.A. Differential correction of lagging-strand replication errors made by DNA polymerases α and δ. Proc. Natl. Acad. Sci. USA 107, 21070–21075 (2010).
Bielinsky, A.K. & Gerbi, S.A. Chromosomal ARS1 has a single leading strand start site. Mol. Cell 3, 477–486 (1999).
Clark, A.B., Lujan, S.A., Kissling, G.E. & Kunkel, T.A. Mismatch repair-independent tandem repeat sequence instability resulting from ribonucleotide incorporation by DNA polymerase ɛ. DNA Repair (Amst.) 10, 476–482 (2011).
Ghodgaonkar, M.M. et al. Ribonucleotides misincorporated into DNA act as strand-discrimination signals in eukaryotic mismatch repair. Mol. Cell 50, 323–332 (2013).
Clausen, A.R., Zhang, S., Burgers, P.M., Lee, M.Y. & Kunkel, T.A. Ribonucleotide incorporation, proofreading and bypass by human DNA polymerase δ. DNA Repair (Amst.) 12, 121–127 (2013).
Williams, J.S. et al. Proofreading of ribonucleotides inserted into DNA by yeast DNA polymerase ɛ. DNA Repair (Amst.) 11, 649–656 (2012).
Kim, N. et al. Mutagenic processing of ribonucleotides in DNA by yeast topoisomerase I. Science 332, 1561–1564 (2011).
Potenski, C.J., Niu, H., Sung, P. & Klein, H.L. Avoidance of ribonucleotide-induced mutations by RNase H2 and Srs2-Exo1 mechanisms. Nature 511, 251–254 (2014).
Allen-Soltero, S., Martinez, S.L., Putnam, C.D. & Kolodner, R.D. A Saccharomyces cerevisiae RNase H2 interaction network functions to suppress genome instability. Mol. Cell. Biol. 34, 1521–1534 (2014).
Reijns, M.A. et al. Enzymatic removal of ribonucleotides from DNA is essential for mammalian genome integrity and development. Cell 149, 1008–1022 (2012).
Hiller, B. et al. Mammalian RNase H2 removes ribonucleotides from DNA to maintain genome integrity. J. Exp. Med. 209, 1419–1426 (2012).
Watt, D.L., Johansson, E., Burgers, P.M. & Kunkel, T.A. Replication of ribonucleotide-containing DNA templates by yeast replicative polymerases. DNA Repair (Amst.) 10, 897–902 (2011).
Clausen, A.R., Murray, M.S., Passer, A.R., Pedersen, L.C. & Kunkel, T.A. Structure-function analysis of ribonucleotide bypass by B family DNA replicases. Proc. Natl. Acad. Sci. USA 110, 16802–16807 (2013).
Lazzaro, F. et al. RNase H and postreplication repair protect cells from ribonucleotides incorporated in DNA. Mol. Cell 45, 99–110 (2012).
Dalgaard, J.Z. Causes and consequences of ribonucleotide incorporation into nuclear DNA. Trends Genet. 28, 592–597 (2012).
Anand, R.P., Lovett, S.T. & Haber, J.E. Break-induced DNA replication. Cold Spring Harb. Perspect. Biol. 5, a010397 (2013).
Foury, F., Roganti, T., Lecrenier, N. & Purnelle, B. The complete sequence of the mitochondrial genome of Saccharomyces cerevisiae. FEBS Lett. 440, 325–331 (1998).
Cerritelli, S.M. & Crouch, R.J. Ribonuclease H: the enzymes in eukaryotes. FEBS J. 276, 1494–1505 (2009).
Grossman, L.I., Watson, R. & Vinograd, J. The presence of ribonucleotides in mature closed-circular mitochondrial DNA. Proc. Natl. Acad. Sci. USA 70, 3339–3343 (1973).
Yang, M.Y. et al. Biased incorporation of ribonucleotides on the mitochondrial L-strand accounts for apparent strand-asymmetric DNA replication. Cell 111, 495–505 (2002).
Kasiviswanathan, R. & Copeland, W.C. Ribonucleotide discrimination and reverse transcription by the human mitochondrial DNA polymerase. J. Biol. Chem. 286, 31490–31500 (2011).
Shadel, G.S. & Clayton, D.A. Mitochondrial DNA maintenance in vertebrates. Annu. Rev. Biochem. 66, 409–435 (1997).
Bowmaker, M. et al. Mammalian mitochondrial DNA replicates bidirectionally from an initiation zone. J. Biol. Chem. 278, 50961–50969 (2003).
Fusté, J.M. et al. Mitochondrial RNA polymerase is needed for activation of the origin of light-strand DNA replication. Mol. Cell 37, 67–78 (2010).
Holt, I.J. & Reyes, A. Human mitochondrial DNA replication. Cold Spring Harb. Perspect. Biol. 4, a012971 (2012).
Cerritelli, S.M. et al. Failure to produce mitochondrial DNA results in embryonic lethality in Rnaseh1 null mice. Mol. Cell 11, 807–815 (2003).
Vengrova, S. & Dalgaard, J.Z. The wild-type Schizosaccharomyces pombe mat1 imprint consists of two ribonucleotides. EMBO Rep. 7, 59–65 (2006).
Koh, K.D., Balachander, S., Hesselberth, J.R., & Storici, F. Ribose-seq: global mapping of ribonucleotides embedded in genomic DNA. Nat. Methods 10.1038/nmeth.3259 (26 January 2015).
Reijns, M.A.M. et al.Lagging strand replication shapes the mutational landscape of the genome. Nature 10.1038/nature14183 (26 January 2015).
Daigaku, Y. et al. A global profile of replicative polymerase usage. Nat. Struct. Mol. Biol. (in the press).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J 17, 10–2 (2011).
Altschul, S.F. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 (1997).
Larkin, M.A. et al. Clustal W and clustal X version 2.0. Bioinformatics 23, 2947–2948 (2007).
Letunic, I. & Bork, P. Interactive Tree Of Life v2: online annotation and display of phylogenetic trees made easy. Nucleic Acids Res. 39, W475–W478 (2011).
Acknowledgements
We thank M. Young and M. Longley for helpful comments on the manuscript. This work was supported by the Division of Intramural Research of the US National Institutes of Health (NIH), National Institute of Environmental Health Sciences (project Z01 ES065070 to T.A.K.) and by NIH grant 2R01GM052319-16A1 to P.A.M.
Author information
Authors and Affiliations
Contributions
A.R.C., D.J.S. and T.A.K. designed the experiments, A.R.C., C.D.O., J.S.W., M.F.C. and E.P.M. performed the experiments, A.R.C., S.A.L., A.B.B., D.C.F., P.A.M. and T.A.K. analyzed the data, T.A.K. wrote the manuscript, and all authors edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Representative 5′ DNA end reads for the previously shown region of chromosome 10.
The region shown matches that of Fig. 2a, bottom panel. All strains are RER-defective (rnh201Δ). The numerical values on the right side of each tract indicate the maximum number of reads in that tract.
Supplementary Figure 3 Evolutionary conservation of the three major eukaryotic nuclear replicases.
(a) Expanded from. Motif A is depicted for the three major eukaryotic nuclear replicases, with residues relevant to this study highlighted in bold and located immediately adjacent to the invariant tyrosine that acts as a steric gate to reduce ribonucleotide incorporation. The number of sequences found for each clade is are shown in parentheses. (b) RNase H2 subunit A catalytic motifs (Chon, H. et al. RNase H2 roles in genome integrity revealed by unlinking its activities. Nucleic Acids Research 41, 3130-3143 (2013)) are similar across the three kingdoms of life. (c) A phylogenetic tree shows RNase H2 subunit A conservation across all major taxa (2396 sequences; less than 1 false positive expected; see Supplementary Table 6).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 and Supplementary Tables 1–4 (PDF 1511 kb)
Supplementary Table 5
List of confirmed and new origins observed by HydEn-seq (XLSX 33 kb)
Supplementary Table 6
RNase H2 subunit A BLAST parameters and hits (XLSX 833 kb)
Supplementary Data Set 1
Uncropped gel image from Figure 1b (PDF 1949 kb)
Rights and permissions
About this article
Cite this article
Clausen, A., Lujan, S., Burkholder, A. et al. Tracking replication enzymology in vivo by genome-wide mapping of ribonucleotide incorporation. Nat Struct Mol Biol 22, 185–191 (2015). https://doi.org/10.1038/nsmb.2957
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2957