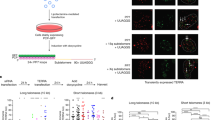
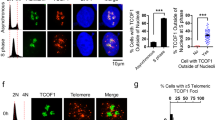
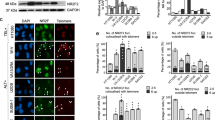
Abstract
Gene silencing by the repressive telomeric chromatin environment, referred to as telomere position effect (TPE), has been well characterized in yeast and depends on telomere length. However, proof of its existence at native human chromosome ends has remained elusive, mainly owing to the paucity of genes near telomeres. The discovery of TERRAs, the telomeric noncoding RNAs transcribed from subtelomeric promoters, paved the way to probing for telomere-length impact on physiological TPE. Using cell lines of various origins, we show that telomere elongation consistently represses TERRA expression. Repression is mediated by increased trimethylated H3K9 density at telomeres and by heterochromatin protein HP1α, with no detectable spreading of the marks beyond the telomeric tract, restricting human TPE to telomere transcription. Our data further support the existence of a negative-feedback mechanism in which longer TERRA molecules repress their own transcription upon telomere elongation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
27 December 2012
In the version of this article initially published, the colors in the key in Figure 7c were incorrectly reversed. Red should have represented telomeres and gray α-satellite regions. The error has been corrected in the HTML and PDF versions of the article.
References
Ottaviani, A., Gilson, E. & Magdinier, F. Telomeric position effect: from the yeast paradigm to human pathologies? Biochimie 90, 93–107 (2008).
Gehring, W.J., Klemenz, R., Weber, U. & Kloter, U. Functional analysis of the white gene of Drosophila by P-factor-mediated transformation. EMBO J. 3, 2077–2085 (1984).
Hazelrigg, T., Levis, R. & Rubin, G.M. Transformation of white locus DNA in Drosophila: dosage compensation, zeste interaction, and position effects. Cell 36, 469–481 (1984).
Perrini, B. et al. HP1 controls telomere capping, telomere elongation, and telomere silencing by two different mechanisms in Drosophila. Mol. Cell 15, 467–476 (2004).
Gottschling, D.E., Aparicio, O.M., Billington, B.L. & Zakian, V.A. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell 63, 751–762 (1990).
Pryde, F.E. & Louis, E.J. Limitations of silencing at native yeast telomeres. EMBO J. 18, 2538–2550 (1999).
Fourel, G., Revardel, E., Koering, C.E. & Gilson, E. Cohabitation of insulators and silencing elements in yeast subtelomeric regions. EMBO J. 18, 2522–2537 (1999).
Nimmo, E.R., Cranston, G. & Allshire, R.C. Telomere-associated chromosome breakage in fission yeast results in variegated expression of adjacent genes. EMBO J. 13, 3801–3811 (1994).
Ofir, R., Wong, A.C., McDermid, H.E., Skorecki, K.L. & Selig, S. Position effect of human telomeric repeats on replication timing. Proc. Natl. Acad. Sci. USA 96, 11434–11439 (1999).
Lou, Z. et al. Telomere length regulates ISG15 expression in human cells. Aging (Albany NY) 1, 608–621 (2009).
Baur, J.A., Zou, Y., Shay, J.W. & Wright, W.E. Telomere position effect in human cells. Science 292, 2075–2077 (2001).
Koering, C.E. et al. Human telomeric position effect is determined by chromosomal context and telomeric chromatin integrity. EMBO Rep. 3, 1055–1061 (2002).
Tennen, R.I., Bua, D.J., Wright, W.E. & Chua, K.F. SIRT6 is required for maintenance of telomere position effect in human cells. Nat. Commun. 2, 433 (2011).
Nergadze, S.G. et al. CpG-island promoters drive transcription of human telomeres. RNA 15, 2186–2194 (2009).
Azzalin, C.M., Reichenbach, P., Khoriauli, L., Giulotto, E. & Lingner, J. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science 318, 798–801 (2007).
Redon, S., Reichenbach, P. & Lingner, J. The non-coding RNA TERRA is a natural ligand and direct inhibitor of human telomerase. Nucleic Acids Res. 38, 5797–5806 (2010).
Schoeftner, S. & Blasco, M.A. Developmentally regulated transcription of mammalian telomeres by DNA-dependent RNA polymerase II. Nat. Cell Biol. 10, 228–236 (2008).
Deng, Z., Norseen, J., Wiedmer, A., Riethman, H. & Lieberman, P.M. TERRA RNA binding to TRF2 facilitates heterochromatin formation and ORC recruitment at telomeres. Mol. Cell 35, 403–413 (2009).
Porro, A., Feuerhahn, S., Reichenbach, P. & Lingner, J. Molecular dissection of telomeric repeat-containing RNA biogenesis unveils the presence of distinct and multiple regulatory pathways. Mol. Cell. Biol. 30, 4808–4817 (2010).
Arnoult, N. et al. Replication timing of human telomeres is chromosome arm-specific, influenced by subtelomeric structures and connected to nuclear localization. PLoS Genet. 6, e1000920 (2010).
Cristofari, G. & Lingner, J. Telomere length homeostasis requires that telomerase levels are limiting. EMBO J. 25, 565–574 (2006).
Takai, K.K., Hooper, S., Blackwood, S., Gandhi, R. & de Lange, T. In vivo stoichiometry of shelterin components. J. Biol. Chem. 285, 1457–1467 (2010).
Mattiussi, M., Tilman, G., Lenglez, S. & Decottignies, A. Human telomerase represses ROS-dependent cellular responses to tumor necrosis factor-α without affecting NF-κB activation. Cell. Signal. 24, 708–717 (2012).
Déjardin, J. & Kingston, R.E. Purification of proteins associated with specific genomic Loci. Cell 136, 175–186 (2009).
Greenwood, J. & Cooper, J.P. Non-coding telomeric and subtelomeric transcripts are differentially regulated by telomeric and heterochromatin assembly factors in fission yeast. Nucleic Acids Res. 40, 2956–2963 (2012).
Shankaranarayana, G.D., Motamedi, M.R., Moazed, D. & Grewal, S.I. Sir2 regulates histone H3 lysine 9 methylation and heterochromatin assembly in fission yeast. Curr. Biol. 13, 1240–1246 (2003).
Rosenfeld, J.A. et al. Determination of enriched histone modifications in non-genic portions of the human genome. BMC Genomics 10, 143 (2009).
O'Sullivan, R.J., Kubicek, S., Schreiber, S.L. & Karlseder, J. Reduced histone biosynthesis and chromatin changes arising from a damage signal at telomeres. Nat. Struct. Mol. Biol. 17, 1218–1225 (2010).
García -Cao, M., O'Sullivan, R., Peters, A.H., Jenuwein, T. & Blasco, M.A. Epigenetic regulation of telomere length in mammalian cells by the Suv39h1 and Suv39h2 histone methyltransferases. Nat. Genet. 36, 94–99 (2004).
Benetti, R., Garcia-Cao, M. & Blasco, M.A. Telomere length regulates the epigenetic status of mammalian telomeres and subtelomeres. Nat. Genet. 39, 243–250 (2007).
Benetti, R., Schoeftner, S., Munoz, P. & Blasco, M.A. Role of TRF2 in the assembly of telomeric chromatin. Cell Cycle 7, 3461–3468 (2008).
Deng, Z., Dheekollu, J., Broccoli, D., Dutta, A. & Lieberman, P.M. The origin recognition complex localizes to telomere repeats and prevents telomere-circle formation. Curr. Biol. 17, 1989–1995 (2007).
Ning, Y. et al. Telomere length and the expression of natural telomeric genes in human fibroblasts. Hum. Mol. Genet. 12, 1329–1336 (2003).
Sharma, G.G. et al. Human heterochromatin protein 1 isoforms HP1(Hsα) and HP1(Hsβ) interfere with hTERT-telomere interactions and correlate with changes in cell growth and response to ionizing radiation. Mol. Cell. Biol. 23, 8363–8376 (2003).
Flynn, R.L. et al. TERRA and hnRNPA1 orchestrate an RPA-to-POT1 switch on telomeric single-stranded DNA. Nature 471, 532–536 (2011).
Muchardt, C. et al. Coordinated methyl and RNA binding is required for heterochromatin localization of mammalian HP1alpha. EMBO Rep. 3, 975–981 (2002).
Maison, C. et al. SUMOylation promotes de novo targeting of HP1α to pericentric heterochromatin. Nat. Genet. 43, 220–227 (2011).
Iglesias, N. et al. Subtelomeric repetitive elements determine TERRA regulation by Rap1/Rif and Rap1/Sir complexes in yeast. EMBO Rep. 12, 587–593 (2011).
Caslini, C., Connelly, J.A., Serna, A., Broccoli, D. & Hess, J.L. MLL associates with telomeres and regulates telomeric repeat-containing RNA transcription. Mol. Cell. Biol. 29, 4519–4526 (2009).
Esnault, G. et al. Transcription factor CTF1 acts as a chromatin domain boundary that shields human telomeric genes from silencing. Mol. Cell. Biol. 29, 2409–2418 (2009).
Donze, D. & Kamakaka, R.T. RNA polymerase III and RNA polymerase II promoter complexes are heterochromatin barriers in Saccharomyces cerevisiae. EMBO J. 20, 520–531 (2001).
Deng, Z., Campbell, A.E. & Lieberman, P.M. TERRA, CpG methylation and telomere heterochromatin: lessons from ICF syndrome cells. Cell Cycle 9, 69–74 (2010).
Tilman, G. et al. Subtelomeric DNA hypomethylation is not required for telomeric sister chromatid exchanges in ALT cells. Oncogene 28, 1682–1693 (2009).
Zhang, L.F. et al. Telomeric RNAs mark sex chromosomes in stem cells. Genetics 182, 685–698 (2009).
Schoeftner, S. et al. Telomere shortening relaxes X chromosome inactivation and forces global transcriptome alterations. Proc. Natl. Acad. Sci. USA 106, 19393–19398 (2009).
Arnoult, N., Saintome, C., Ourliac-Garnier, I., Riou, J.F. & Londono-Vallejo, A. Human POT1 is required for efficient telomere C-rich strand replication in the absence of WRN. Genes Dev. 23, 2915–2924 (2009).
Willard, H.F., Smith, K.D. & Sutherland, J. Isolation and characterization of a major tandem repeat family from the human X chromosome. Nucleic Acids Res. 11, 2017–2033 (1983).
Draskovic, I. et al. Probing PML body function in ALT cells reveals spatiotemporal requirements for telomere recombination. Proc. Natl. Acad. Sci. USA 106, 15726–15731 (2009).
Acknowledgements
We are grateful to J. Lingner and G. Cristofari (École polytechnique fédérale de Lausanne, Lausanne, Switzerland), T. de Lange (The Rockefeller University, New York, New York, USA), F. Fuks (Université Libre de Bruxelles, Brussels, Belgium), H. Antoine-Poirel (Université catholique de Louvain, Brussels, Belgium) and C. Heirman (Vrije Universiteit Brussel, Brussels, Belgium) for the generous gift of cell lines and plasmids. We thank G. Tilman, A. Loriot and C. De Smet for useful comments on the manuscript and N. Dauguet for technical assistance with flow cytometry. We warmly thank the de Duve Institute for constant support. This work was supported by the Belgian Fonds National de la Recherche Scientifique: Mandat d'Impulsion Scientifique MIS F.4511.09 (to N.A. and A.D.) and by the Fonds pour la formation à la Recherche dans l'Industrie et dans l'Agriculture (FRIA) fellowship FNRS 1.E127.10 (to A.V.B.).
Author information
Authors and Affiliations
Contributions
A.D. and N.A. conceived and planned the project, and wrote the manuscript. N.A. and A.V.B. performed the experiments. N.A., A.V.B. and A.D. analyzed the results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Tables 1 and 2 (PDF 4123 kb)
Rights and permissions
About this article
Cite this article
Arnoult, N., Van Beneden, A. & Decottignies, A. Telomere length regulates TERRA levels through increased trimethylation of telomeric H3K9 and HP1α. Nat Struct Mol Biol 19, 948–956 (2012). https://doi.org/10.1038/nsmb.2364
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2364
This article is cited by
-
Genome-wide analysis of sex-specific differences in the mother–child PELAGIE cohort exposed to organophosphate metabolites
Scientific Reports (2023)
-
Sexual Dimorphism in Telomere Length in Childhood Autism
Journal of Autism and Developmental Disorders (2023)
-
Regulation potential of transcribed simple repeated sequences in developing neurons
Human Genetics (2023)
-
BRCA1 binds TERRA RNA and suppresses R-Loop-based telomeric DNA damage
Nature Communications (2021)
-
Gametes deficient for Pot1 telomere binding proteins alter levels of telomeric foci for multiple generations
Communications Biology (2021)