Abstract
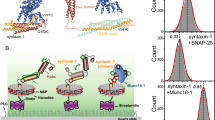
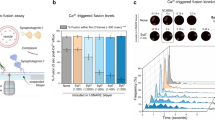
The crystal structure of complexin bound to a prefusion SNAREpin mimetic shows that the accessory helix extends away from the SNAREpin in an 'open' conformation, binding another SNAREpin and inhibiting its assembly, to clamp fusion. In contrast, the accessory helix in the postfusion complex parallels the SNARE complex in a 'closed' conformation. Here we use targeted mutations, FRET spectroscopy and a functional assay that reconstitutes Ca2+-triggered exocytosis to show that the conformational switch from open to closed in complexin is needed for synaptotagmin-Ca2+ to trigger fusion. Triggering fusion requires the zippering of three crucial aspartate residues in the switch region (residues 64–68) of v-SNARE. Conformational switching in complexin is integral to clamp release and is probably triggered when its accessory helix is released from its trans-binding to the neighboring SNAREpin, allowing the v-SNARE to complete zippering and open a fusion pore.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Söllner, T. et al. SNAP receptors implicated in vesicle targeting and fusion. Nature 362, 318–324 (1993).
Hu, C. et al. Fusion of cells by flipped SNAREs. Science 300, 1745–1749 (2003).
McNew, J.A. et al. Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature 407, 153–159 (2000).
Weber, T. et al. SNAREpins: minimal machinery for membrane fusion. Cell 92, 759–772 (1998).
Sutton, R.B., Fasshauer, D., Jahn, R. & Brunger, A.T. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature 395, 347–353 (1998).
Karatekin, E. et al. A fast, single-vesicle fusion assay mimics physiological SNARE requirements. Proc. Natl. Acad. Sci. USA 107, 3517–3521 (2010).
Perin, M.S., Fried, V.A., Mignery, G.A., Jahn, R. & Sudhof, T.C. Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature 345, 260–263 (1990).
Brose, N., Petrenko, A.G., Sudhof, T.C. & Jahn, R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science 256, 1021–1025 (1992).
Fernández-Chacón, R. et al. Synaptotagmin I functions as a calcium regulator of release probability. Nature 410, 41–49 (2001).
Geppert, M. et al. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell 79, 717–727 (1994).
Pang, Z.P., Shin, O.H., Meyer, A.C., Rosenmund, C. & Sudhof, T.C. A gain-of-function mutation in synaptotagmin-1 reveals a critical role of Ca2+-dependent soluble N-ethylmaleimide-sensitive factor attachment protein receptor complex binding in synaptic exocytosis. J. Neurosci. 26, 12556–12565 (2006).
Ishizuka, T., Saisu, H., Odani, S. & Abe, T. Synaphin: a protein associated with the docking/fusion complex in presynaptic terminals. Biochem. Biophys. Res. Commun. 213, 1107–1114 (1995).
McMahon, H.T., Missler, M., Li, C. & Sudhof, T.C. Complexins: cytosolic proteins that regulate SNAP receptor function. Cell 83, 111–119 (1995).
Giraudo, C.G., Eng, W.S., Melia, T.J. & Rothman, J.E. A clamping mechanism involved in SNARE-dependent exocytosis. Science 313, 676–680 (2006).
Giraudo, C.G. et al. Alternative zippering as an on-off switch for SNARE-mediated fusion. Science 323, 512–516 (2009).
Maximov, A., Tang, J., Yang, X., Pang, Z.P. & Sudhof, T.C. Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science 323, 516–521 (2009).
Xue, M. et al. Distinct domains of complexin I differentially regulate neurotransmitter release. Nat. Struct. Mol. Biol. 14, 949–958 (2007).
Xue, M. et al. Tilting the balance between facilitatory and inhibitory functions of mammalian and Drosophila Complexins orchestrates synaptic vesicle exocytosis. Neuron 64, 367–380 (2009).
Südhof, T.C. & Rothman, J.E. Membrane fusion: grappling with SNARE and SM proteins. Science 323, 474–477 (2009).
Li, F. et al. Complexin activates and clamps SNAREpins by a common mechanism involving an intermediate energetic state. Nat. Struct. Mol. Biol. doi:10.1038/nsmb.2102 (2011).
Kümmel, D. et al. Complexin cross-links prefusion SNAREs into a zigzag array. Nat. Struct. Mol. Biol. doi:10.1038/nsmb.2101 (2011).
Hua, S.Y. & Charlton, M.P. Activity-dependent changes in partial VAMP complexes during neurotransmitter release. Nat. Neurosci. 2, 1078–1083 (1999).
Reim, K. et al. Complexins regulate a late step in Ca2+-dependent neurotransmitter release. Cell 104, 71–81 (2001).
Tang, J. et al. A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell 126, 1175–1187 (2006).
Chen, X. et al. Three-dimensional structure of the complexin/SNARE complex. Neuron 33, 397–409 (2002).
Bracher, A., Kadlec, J., Betz, H. & Weissenhorn, W. X-ray structure of a neuronal complexin-SNARE complex from squid. J. Biol. Chem. 277, 26517–26523 (2002).
Yang, X., Kaeser-Woo, Y.J., Pang, Z.P., Xu, W. & Sudhof, T.C. Complexin clamps asynchronous release by blocking a secondary Ca2+ sensor via its accessory alpha helix. Neuron 68, 907–920 (2010).
Shao, X., Fernandez, I., Sudhof, T.C. & Rizo, J. Solution structures of the Ca2+-free and Ca2+-bound C2A domain of synaptotagmin I: does Ca2+ induce a conformational change? Biochemistry 37, 16106–16115 (1998).
Radhakrishnan, A., Stein, A., Jahn, R. & Fasshauer, D. The Ca2+ affinity of synaptotagmin 1 is markedly increased by a specific interaction of its C2B domain with phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 284, 25749–25760 (2009).
Vrljic, M. et al. Molecular mechanism of the synaptotagmin-SNARE interaction in Ca2+-triggered vesicle fusion. Nat. Struct. Mol. Biol. 17, 325–331 (2010).
Ubach, J., Zhang, X., Shao, X., Sudhof, T.C. & Rizo, J. Ca2+ binding to synaptotagmin: how many Ca2+ ions bind to the tip of a C2-domain? EMBO J. 17, 3921–3930 (1998).
Bai, J., Tucker, W.C. & Chapman, E.R. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nat. Struct. Mol. Biol. 11, 36–44 (2004).
Chapman, E.R. & Davis, A.F. Direct interaction of a Ca2+-binding loop of synaptotagmin with lipid bilayers. J. Biol. Chem. 273, 13995–14001 (1998).
McMahon, H.T., Kozlov, M.M. & Martens, S. Membrane curvature in synaptic vesicle fusion and beyond. Cell 140, 601–605 (2010).
Xue, M. et al. Binding of the complexin N terminus to the SNARE complex potentiates synaptic-vesicle fusogenicity. Nat. Struct. Mol. Biol. 17, 568–575 (2010).
Yoon, T.Y. et al. Complexin and Ca2+ stimulate SNARE-mediated membrane fusion. Nat. Struct. Mol. Biol. 15, 707–713 (2008).
Sabatini, B.L. & Regehr, W.G. Timing of neurotransmission at fast synapses in the mammalian brain. Nature 384, 170–172 (1996).
Evans, E. Probing the relation between force–lifetime–and chemistry in single molecular bonds. Annu. Rev. Biophys. Biomol. Struct. 30, 105–128 (2001).
Scott, B.L. et al. Liposome fusion assay to monitor intracellular membrane fusion machines. Methods Enzymol. 372, 274–300 (2003).
Ji, H. et al. Protein determinants of SNARE-mediated lipid mixing. Biophys. J. 99, 553–560 (2010).
Giraudo, C.G. et al. Distinct domains of complexins bind SNARE complexes and clamp fusion in vitro. J. Biol. Chem. 283, 21211–21219 (2008).
Acknowledgements
We wish to thank T. Melia (Yale University) for critical reading of the manuscript. This work was supported by US National Institutes of Health grants to J.E.R. and K.M.R., an Agence Nationale de la Recherche (ANR) Physique et Chimie du Vivant (PCV) grant to F.P., and a grant from the Deutsche Forschungsgemeinschaft to D.K.
Author information
Authors and Affiliations
Contributions
S.S.K., D.T.R. and D.K. did the mutagenesis and protein purification, S.S.K. and D.T.R. carried out the fluorescence measurements, F.L. took the ITC measurements and C.G.G. did the cell-cell fusion assay. L.K., S.B. and J.C. provided technical assistance. Results were analyzed and discussed by all authors. The manuscript was prepared by S.S.K., D.T.R., K.M.R., F.P. and J.E.R.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 (PDF 846 kb)
Rights and permissions
About this article
Cite this article
Krishnakumar, S., Radoff, D., Kümmel, D. et al. A conformational switch in complexin is required for synaptotagmin to trigger synaptic fusion. Nat Struct Mol Biol 18, 934–940 (2011). https://doi.org/10.1038/nsmb.2103
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2103
This article is cited by
-
Structural basis for the clamping and Ca2+ activation of SNARE-mediated fusion by synaptotagmin
Nature Communications (2019)
-
v-SNARE function in chromaffin cells
Pflügers Archiv - European Journal of Physiology (2018)
-
Genome-wide association study revealed genomic regions related to white/red earlobe color trait in the Rhode Island Red chickens
BMC Genetics (2016)
-
Nanodisc-cell fusion: control of fusion pore nucleation and lifetimes by SNARE protein transmembrane domains
Scientific Reports (2016)
-
Molecular origins of synaptotagmin 1 activities on vesicle docking and fusion pore opening
Scientific Reports (2015)