Abstract
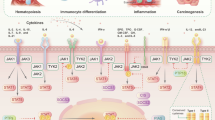
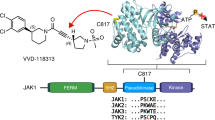
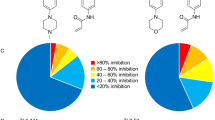
Human JAK2 tyrosine kinase mediates signaling through numerous cytokine receptors. The JAK2 JH2 domain functions as a negative regulator and is presumed to be a catalytically inactive pseudokinase, but the mechanism(s) for its inhibition of JAK2 remains unknown. Mutations in JH2 lead to increased JAK2 activity, contributing to myeloproliferative neoplasms (MPNs). Here we show that JH2 is a dual-specificity protein kinase that phosphorylates two negative regulatory sites in JAK2: Ser523 and Tyr570. Inactivation of JH2 catalytic activity increased JAK2 basal activity and downstream signaling. Notably, different MPN mutations abrogated JH2 activity in cells, and in MPN (V617F) patient cells phosphorylation of Tyr570 was reduced, suggesting that loss of JH2 activity contributes to the pathogenesis of MPNs. These results identify the catalytic activity of JH2 as a previously unrecognized mechanism to control basal activity and signaling of JAK2.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Gadina, M. et al. Signaling by type I and II cytokine receptors: ten years after. Curr. Opin. Immunol. 13, 363–373 (2001).
Imada, K. & Leonard, W.J. The Jak-STAT pathway. Mol. Immunol. 37, 1–11 (2000).
Silvennoinen, O. et al. Structure of the murine Jak2 protein-tyrosine kinase and its role in interleukin 3 signal transduction. Proc. Natl. Acad. Sci. USA 90, 8429–8433 (1993).
Yamaoka, K. et al. The Janus kinases (Jaks). Genome Biol. 5, 253 (2004).
Ishida-Takahashi, R. et al. Phosphorylation of Jak2 on Ser(523) inhibits Jak2-dependent leptin receptor signaling. Mol. Cell. Biol. 26, 4063–4073 (2006).
Matsuda, T., Feng, J., Witthuhn, B.A., Sekine, Y. & Ihle, J.N. Determination of the transphosphorylation sites of Jak2 kinase. Biochem. Biophys. Res. Commun. 325, 586–594 (2004).
Mazurkiewicz-Munoz, A.M. et al. Phosphorylation of JAK2 at serine 523: a negative regulator of JAK2 that is stimulated by growth hormone and epidermal growth factor. Mol. Cell. Biol. 26, 4052–4062 (2006).
Robertson, S.A. et al. Regulation of Jak2 function by phosphorylation of Tyr317 and Tyr637 during cytokine signaling. Mol. Cell. Biol. 29, 3367–3378 (2009).
Feener, E.P., Rosario, F., Dunn, S.L., Stancheva, Z. & Myers, M.G. Jr. Tyrosine phosphorylation of Jak2 in the JH2 domain inhibits cytokine signaling. Mol. Cell. Biol. 24, 4968–4978 (2004).
Argetsinger, L.S. et al. Autophosphorylation of JAK2 on tyrosines 221 and 570 regulates its activity. Mol. Cell. Biol. 24, 4955–4967 (2004).
Argetsinger, L.S. et al. Tyrosines 868, 966, and 972 in the kinase domain of JAK2 are autophosphorylated and required for maximal JAK2 kinase activity. Mol. Endocrinol. 24, 1062–1076 (2010).
Shuai, K. & Liu, B. Regulation of JAK-STAT signalling in the immune system. Nat. Rev. Immunol. 3, 900–911 (2003).
Saharinen, P., Takaluoma, K. & Silvennoinen, O. Regulation of the Jak2 tyrosine kinase by its pseudokinase domain. Mol. Cell. Biol. 20, 3387–3395 (2000).
Saharinen, P. & Silvennoinen, O. The pseudokinase domain is required for suppression of basal activity of Jak2 and Jak3 tyrosine kinases and for cytokine-inducible activation of signal transduction. J. Biol. Chem. 277, 47954–47963 (2002).
Saharinen, P., Vihinen, M. & Silvennoinen, O. Autoinhibition of Jak2 tyrosine kinase is dependent on specific regions in its pseudokinase domain. Mol. Biol. Cell 14, 1448–1459 (2003).
Haan, C., Behrmann, I. & Haan, S. Perspectives for the use of structural information and chemical genetics to develop inhibitors of Janus kinases. J. Cell. Mol. Med. 14, 504–527 (2010).
Baxter, E.J. et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 365, 1054–1061 (2005).
James, C. et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 434, 1144–1148 (2005).
Kralovics, R. et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Engl. J. Med. 352, 1779–1790 (2005).
Chen, M. et al. Complex effects of naturally occurring mutations in the JAK3 pseudokinase domain: evidence for interactions between the kinase and pseudokinase domains. Mol. Cell. Biol. 20, 947–956 (2000).
Lucet, I.S. et al. The structural basis of Janus kinase 2 inhibition by a potent and specific pan-Janus kinase inhibitor. Blood 107, 176–183 (2006).
Boggon, T.J., Li, Y., Manley, P.W. & Eck, M.J. Crystal structure of the Jak3 kinase domain in complex with a staurosporine analog. Blood 106, 996–1002 (2005).
Boudeau, J., Miranda-Saavedra, D., Barton, G.J. & Alessi, D.R. Emerging roles of pseudokinases. Trends Cell Biol. 16, 443–452 (2006).
Hubbard, S.R. & Till, J.H. Protein tyrosine kinase structure and function. Annu. Rev. Biochem. 69, 373–398 (2000).
Hall, T. et al. Expression, purification, characterization and crystallization of non- and phosphorylated states of JAK2 and JAK3 kinase domain. Protein Expr. Purif. 69, 54–63 (2010).
Scott, L.M. et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N. Engl. J. Med. 356, 459–468 (2007).
Bercovich, D. et al. Mutations of JAK2 in acute lymphoblastic leukaemias associated with Down's syndrome. Lancet 372, 1484–1492 (2008).
Zeqiraj, E. & van Aalten, D.M. Pseudokinases-remnants of evolution or key allosteric regulators? Curr. Opin. Struct. Biol. 20, 772–781 (2010).
Scheeff, E.D., Eswaran, J., Bunkoczi, G., Knapp, S. & Manning, G. Structure of the pseudokinase VRK3 reveals a degraded catalytic site, a highly conserved kinase fold, and a putative regulatory binding site. Structure 17, 128–138 (2009).
Mukherjee, K. et al. CASK functions as a Mg2+-independent neurexin kinase. Cell 133, 328–339 (2008).
Eswaran, J. et al. Structure and functional characterization of the atypical human kinase haspin. Proc. Natl. Acad. Sci. USA 106, 20198–20203 (2009).
Min, X., Lee, B.H., Cobb, M.H. & Goldsmith, E.J. Crystal structure of the kinase domain of WNK1, a kinase that causes a hereditary form of hypertension. Structure 12, 1303–1311 (2004).
Shi, F., Telesco, S.E., Liu, Y., Radhakrishnan, R. & Lemmon, M.A. ErbB3/HER3 intracellular domain is competent to bind ATP and catalyze autophosphorylation. Proc. Natl. Acad. Sci. USA 107, 7692–7697 (2010).
Zeqiraj, E., Filippi, B.M., Deak, M., Alessi, D.R. & van Aalten, D.M. Structure of the LKB1-STRAD-MO25 complex reveals an allosteric mechanism of kinase activation. Science 326, 1707–1711 (2009).
Jura, N., Shan, Y., Cao, X., Shaw, D.E. & Kuriyan, J. Structural analysis of the catalytically inactive kinase domain of the human EGF receptor 3. Proc. Natl. Acad. Sci. USA 106, 21608–21613 (2009).
Santos, F.P. & Verstovsek, S. JAK2 inhibitors: what's the true therapeutic potential? Blood Rev. 25, 53–63 (2011).
Shevchenko, A., Tomas, H., Havlis, J., Olsen, J.V. & Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 1, 2856–2860 (2006).
Thingholm, T.E., Jorgensen, T.J., Jensen, O.N. & Larsen, M.R. Highly selective enrichment of phosphorylated peptides using titanium dioxide. Nat. Protoc. 1, 1929–1935 (2006).
Ye, J. et al. Optimized IMAC-IMAC protocol for phosphopeptide recovery from complex biological samples. J. Proteome Res. 9, 3561–3573 (2010).
Acknowledgements
We thank M. Myers (University of Michigan Medical School) for reagents (anti-pSer523 and anti-pTyr570 specific antibodies), E. Koskenalho, P. Kosonen and M. Lehtinen for technical assistance, and the Biocenter Finland protein production platform (V. Hytönen and U. Kiiskinen) for technological support. This study was supported by the Medical Research Council of Academy of Finland (O.S.), the Sigrid Juselius Foundation (O.S.), the Finnish Cancer Foundation (O.S.), the EU Research Training Network ReceptEur (O.S.), Science Center, Competitive Research Funding and Centre of Laboratory Medicine of the Tampere University Hospital (O.S.), the Tampere Tuberculosis Foundation (O.S.), US National Institutes of Health core grant CA016087 (T.A.N.), the Danish Research Agency and the Danish National Research Foundation (Centre for Epigenetics) (C.Y. and O.N.J.).
Author information
Authors and Affiliations
Contributions
D.U. performed the experiments and wrote the paper. O.S. and S.R.H. designed the experiments and wrote the paper. J.W. performed the in vitro experiments with recombinant proteins. T.P. and Y.N. performed the mutagenesis experiments in mammalian cells. C.Y., O.N.J., T.A.N. and C.-F.X. performed the experiments for MS analysis. R.C.S. performed the experiments for clinical sample analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Methods (PDF 13161 kb)
Rights and permissions
About this article
Cite this article
Ungureanu, D., Wu, J., Pekkala, T. et al. The pseudokinase domain of JAK2 is a dual-specificity protein kinase that negatively regulates cytokine signaling. Nat Struct Mol Biol 18, 971–976 (2011). https://doi.org/10.1038/nsmb.2099
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2099
This article is cited by
-
Double-edged sword of JAK/STAT signaling pathway in viral infections: novel insights into virotherapy
Cell Communication and Signaling (2023)
-
Pathways involved in pony body size development
BMC Genomics (2021)
-
New insights into IL-6 family cytokines in metabolism, hepatology and gastroenterology
Nature Reviews Gastroenterology & Hepatology (2021)
-
Prospects for pharmacological targeting of pseudokinases
Nature Reviews Drug Discovery (2019)
-
Computational algorithms for in silico profiling of activating mutations in cancer
Cellular and Molecular Life Sciences (2019)