Abstract
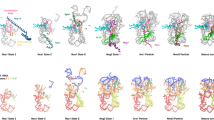
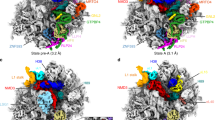
As translation proceeds, the nascent polypeptide chain passes through a tunnel in the large ribosomal subunit. Although this ribosomal exit tunnel was once thought only to be a passive conduit for the growing nascent chain, accumulating evidence suggests that it may in fact play a more active role in regulating translation and initial protein folding events. Here we have determined single-particle cryo–electron microscopy reconstructions of eukaryotic 80S ribosomes containing nascent chains with high α-helical propensity located within the exit tunnel. The maps enable direct visualization of density for helices as well as allowing the sites of interaction with the tunnel wall components to be elucidated. In particular regions of the tunnel, the nascent chain adopts distinct conformations and establishes specific contacts with tunnel components, both ribosomal RNA and proteins, that have been previously implicated in nascent chain–ribosome interaction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Frank, J. et al. A model of protein synthesis based on cryo–electron microscopy of the E. coli ribosome. Nature 376, 441–444 (1995).
Ban, N., Nissen, P., Hansen, J., Moore, P.B. & Steitz, T.A. The complete atomic structure of the large ribosomal subunit at 2.4 Å resolution. Science 289, 905–920 (2000).
Morgan, D.G. et al. A comparison of the yeast and rabbit 80 S ribosome reveals the topology of the nascent chain exit tunnel, inter-subunit bridges and mammalian rRNA expansion segments. J. Mol. Biol. 301, 301–321 (2000).
Halic, M. et al. Signal recognition particle receptor exposes the ribosomal translocon binding site. Science 312, 745–747 (2006).
Lu, J. & Deutsch, C. Electrostatics in the ribosomal tunnel modulate chain elongation rates. J. Mol. Biol. 384, 73–86 (2008).
Lu, J., Kobertz, W.R. & Deutsch, C. Mapping the electrostatic potential within the ribosomal exit tunnel. J. Mol. Biol. 371, 1378–1391 (2007).
Tenson, T. & Ehrenberg, M. Regulatory nascent peptides in the ribosomal tunnel. Cell 108, 591–594 (2002).
Gong, F. & Yanofsky, C. Instruction of translating ribosome by nascent peptide. Science 297, 1864–1867 (2002).
Nakatogawa, H. & Ito, K. The ribosomal exit tunnel functions as a discriminating gate. Cell 108, 629–636 (2002).
Vazquez-Laslop, N., Thum, C. & Mankin, A.S. Molecular mechanism of drug-dependent ribosome stalling. Mol. Cell 30, 190–202 (2008).
Voss, N.R., Gerstein, M., Steitz, T.A. & Moore, P.B. The geometry of the ribosomal polypeptide exit tunnel. J. Mol. Biol. 360, 893–906 (2006).
Hardesty, B. & Kramer, G. Folding of a nascent peptide on the ribosome. Prog. Nucleic Acid Res. Mol. Biol. 66, 41–66 (2001).
Woolhead, C.A., McCormick, P.J. & Johnson, A.E. Nascent membrane and secretory proteins differ in FRET-detected folding far inside the ribosome and in their exposure to ribosomal proteins. Cell 116, 725–736 (2004).
Kosolapov, A., Tu, L., Wang, J. & Deutsch, C. Structure acquisition of the T1 domain of Kv1.3 during biogenesis. Neuron 44, 295–307 (2004).
Lu, J. & Deutsch, C. Folding zones inside the ribosomal exit tunnel. Nat. Struct. Mol. Biol. 12, 1123–1129 (2005).
Lu, J. & Deutsch, C. Secondary structure formation of a transmembrane segment in Kv channels. Biochemistry 44, 8230–8243 (2005).
Woolhead, C.A., Johnson, A.E. & Bernstein, H.D. Translation arrest requires two-way communication between a nascent polypeptide and the ribosome. Mol. Cell 22, 587–598 (2006).
Halic, M. et al. Structure of the signal recognition particle interacting with the elongation-arrested ribosome. Nature 427, 808–814 (2004).
Halic, M. et al. Following the signal sequence from ribosomal tunnel exit to signal recognition particle. Nature 444, 507–511 (2006).
Marqusee, S. & Baldwin, R.L. Helix stabilization by Glu−.Lys+ salt bridges in short peptides of de novo design. Proc. Natl. Acad. Sci. USA 84, 8898–8902 (1987).
Arai, R., Ueda, H., Kitayama, A., Kamiya, N. & Nagamune, T. Design of the linkers which effectively separate domains of a bifunctional fusion protein. Protein Eng. 14, 529–532 (2001).
Sicheri, F. & Yang, D.S. Ice-binding structure and mechanism of an antifreeze protein from winter flounder. Nature 375, 427–431 (1995).
Liepinsh, E. et al. Solution structure of a hydrophobic analogue of the winter flounder antifreeze protein. Eur. J. Biochem. 269, 1259–1266 (2002).
Schmeing, T.M., Huang, K.S., Strobel, S.A. & Steitz, T.A. An induced-fit mechanism to promote peptide bond formation and exclude hydrolysis of peptidyl-tRNA. Nature 438, 520–524 (2005).
Cruz-Vera, L.R., Rajagopal, S., Squires, C. & Yanofsky, C. Features of ribosome-peptidyl-tRNA interactions essential for tryptophan induction of tna operon expression. Mol. Cell 19, 333–343 (2005).
Kosolapov, A. & Deutsch, C. Tertiary interactions within the ribosomal exit tunnel. Nat. Struct. Mol. Biol. 16, 405–411 (2009).
Liao, S., Lin, J., Do, H. & Johnson, A.E. Both lumenal and cytosolic gating of the aqueous ER translocon pore are regulated from inside the ribosome during membrane protein integration. Cell 90, 31–41 (1997).
Berndt, U., Oellerer, S., Zhang, Y., Johnson, A.E. & Rospert, S. A signal-anchor sequence stimulates signal recognition particle binding to ribosomes from inside the exit tunnel. Proc. Natl. Acad. Sci. USA 106, 1398–1403 (2009).
Mingarro, I., Nilsson, I., Whitley, P. & von Heijne, G. Different conformations of nascent polypeptides during translocation across the ER membrane. BMC Cell Biol. 1, 3 (2000).
Erickson, A.H. & Blobel, G. Cell-free translation of messenger RNA in a wheat germ system. Methods Enzymol. 96, 38–50 (1983).
Wagenknecht, T., Frank, J., Boublik, M., Nurse, K. & Ofengand, J. Direct localization of the tRNA-anticodon interaction site on the Escherichia coli 30 S ribosomal subunit by electron microscopy and computerized image averaging. J. Mol. Biol. 203, 753–760 (1988).
Mindell, J.A. & Grigorieff, N. Accurate determination of local defocus and specimen tilt in electron microscopy. J. Struct. Biol. 142, 334–347 (2003).
Frank, J. Three-dimensional electron microscopy of macromolecular assemblies. in Three-dimensional Electron Microscopy of Macromolecular Assemblies (Academic Press, 1996).
Jossinet, F. & Westhof, E. Sequence to structure (S2S): display, manipulate and interconnect RNA data from sequence to structure. Bioinformatics 21, 3320–3321 (2005).
Selmer, M. et al. Structure of the 70S ribosome complexed with mRNA and tRNA. Science 313, 1935–1942 (2006).
Schuwirth, B.S. et al. Structures of the bacterial ribosome at 3.5 Å resolution. Science 310, 827–834 (2005).
Massire, C. & Westhof, E. MANIP: an interactive tool for modelling RNA. J. Mol. Graph. Model. 16, 197–205, 255–197 (1998).
Yang, H. et al. Tools for the automatic identification and classification of RNA base pairs. Nucleic Acids Res. 31, 3450–3460 (2003).
Trabuco, L.G., Villa, E., Mitra, K., Frank, J. & Schulten, K. Flexible fitting of atomic structures into electron microscopy maps using molecular dynamics. Structure 16, 673–683 (2008).
Notredame, C., Higgins, D.G. & Heringa, J. T-Coffee: a novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 302, 205–217 (2000).
Eswar, N. et al. Comparative protein structure modeling using Modeller. Curr. Protoc. Bioinformatics 5, 5.6 (2006).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Schmeing, T.M., Huang, K.S., Kitchen, D.E., Strobel, S.A. & Steitz, T.A. Structural insights into the roles of water and the 2′ hydroxyl of the P site tRNA in the peptidyl transferase reaction. Mol. Cell 20, 437–448 (2005).
Pettersen, E.F. et al. UCSF Chimera: a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Acknowledgements
We would like to thank B. Beatrix for help with the wheat germ translation system and J. Buerger for help with the electron microscopy. This research was supported by Federation of European Biochemical Societies and Knut och Alice Wallenbergs Stiftelse postdoctoral fellowships (to S.B.), grants from the Deutsche Forschungsgemeinschaft SFB594 and SFB646 (to R.B.), SFB740 (to T.M.) and WI3285/1-1 (to D.N.W.) and by the European Union and Senatsverwaltung für Wissenschaft, Forschung und Kultur Berlin.
Author information
Authors and Affiliations
Contributions
S.B., M.G. and R.B. designed research; S.B. prepared the complexes and collected data; O.B. and T.M. helped with data collection; S.B., M.G. and M.H. processed datasets; J.-P.A., A.J. and D.N.W. prepared models; S.B., D.N.W. and R.B. analyzed results, prepared figures and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 4913 kb)
Rights and permissions
About this article
Cite this article
Bhushan, S., Gartmann, M., Halic, M. et al. α-Helical nascent polypeptide chains visualized within distinct regions of the ribosomal exit tunnel. Nat Struct Mol Biol 17, 313–317 (2010). https://doi.org/10.1038/nsmb.1756
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.1756