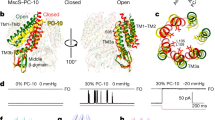
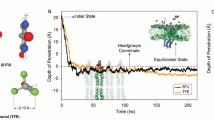
Abstract
In mechanosensitive (MS) channels, gating is initiated by changes in intra-bilayer pressure profiles originating from bilayer deformation. Here we evaluated two physical mechanisms as triggers of MS channel gating: the energetic cost of protein–bilayer hydrophobic mismatches and the geometric consequences of bilayer intrinsic curvature. Structural changes in the Escherichia coli large MS channel (MscL) were studied under nominally zero transbilayer pressures using both patch clamp and EPR spectroscopic approaches. Changes in membrane intrinsic curvature induced by the external addition of lysophosphatidylcholine (LPC) generated massive spectroscopic changes in the narrow constriction that forms the channel 'gate', trapping the channel in the fully open state. Hydrophobic mismatch alone was unable to open the channel, but decreasing bilayer thickness lowered MscL activation energy, stabilizing a structurally distinct closed channel intermediate. We propose that the mechanism of mechanotransduction in MS channels is defined by both local and global asymmetries in the transbilayer pressure profile at the lipid–protein interface.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Sukharev, S.I., Blount, P., Martinac, B. & Kung, C. Mechanosensitive channels of Escherichia coli: the MscL gene, protein, and activities. Annu. Rev. Physiol. 59, 633–657 (1997).
Booth, I.R. & Louis, P. Managing hypoosmotic stress: aquaporins and mechanosensitive channels in Escherichia coli. Curr. Opin. Microbiol. 2, 166–169 (1999).
Wood, J.M. Osmosensing by bacteria: signals and membrane-based sensors. Microbiol. Mol. Biol. Rev. 63, 230–262 (1999).
Hamill, O.P. & Martinac, B. Molecular basis of mechanotransduction in living cells. Physiol. Rev. 81, 685–740 (2001).
Martinac, B. Mechanosensitive channels in prokaryotes. Cell. Physiol. Biochem. 11, 61–76 (2001).
Martinac, B., Buechner, M., Delcour, A.H., Adler, J. & Kung, C. Pressure-sensitive ion channel in Escherichia coli. Proc. Natl. Acad. Sci. USA 84, 2297–2301 (1987).
Sukharev, S.I., Martinac, B., Arshavsky, V.Y. & Kung, C. Two types of mechanosensitive channels in the Escherichia coli cell envelope: solubilization and functional reconstitution. Biophys. J. 65, 177–183 (1993).
Sukharev, S.I., Blount, P., Martinac, B., Blattner, F.R. & Kung, C. A large-conductance mechanosensitive channel in E. coli encoded by MscL alone. Nature 368, 265–268 (1994).
Chang, G., Spencer, R.H., Lee, A.T., Barclay, M.T. & Rees, D.C. Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science 282, 2220–2226 (1998).
Berrier, C., Coulombe, A., Houssin, C. & Ghazi, A. A patch-clamp study of ion channels of inner and outer membranes and of contact zones of E. coli, fused into giant liposomes. Pressure-activated channels are localized in the inner membrane. FEBS Lett. 259, 27–32 (1989).
Cantor, R.S. Lateral pressures in cell membranes: a mechanism for modulation of protein function. J. Phys. Chem. B 101, 1723–1725 (1997).
Cantor, R.S. Lipid composition and the lateral pressure profile in bilayers. Biophys J. 76, 2625–2639 (1999).
Mouritsen, O.G. & Bloom, M. Mattress model of lipid-protein interactions in membranes. Biophys. J. 46, 141–153 (1984).
Gruner, S. in Biologically Inspired Physics (ed. Peliti, L.) 127–135 (Plenum, New York; 1991).
Israelachvili, J. Intermolecular and Surface Forces (Academic Press, New York; 1992).
Killian, J.A. Hydrophobic mismatch between proteins and lipids in membranes. Biochim. Biophys. Acta 1376, 401–415 (1998).
Cornea, R.L. & Thomas, D.D. Effects of membrane thickness on the molecular dynamics and enzymatic activity of reconstituted Ca-ATPase. Biochemistry 33, 2912–2920 (1994).
Galbraith, T.P. & Wallace, B.A. Phospholipid chain length alters the equilibrium between pore and channel forms of gramicidin. Faraday Discuss. 111, 159–164 (1998).
Lundbaek, J.A. & Andersen, O.S. Spring constants for channel-induced lipid bilayer deformations. Estimates using gramicidin channels. Biophys. J. 76, 889–895 (1999).
Dumas, F., Tocanne, J.F., Leblanc, G. & Lebrun, M.C. Consequences of hydrophobic mismatch between lipids and melibiose permease on melibiose transport. Biochemistry 39, 4846–4854 (2000).
Lewis, B.A. & Engelman, D.M. Lipid bilayer thickness varies linearly with acyl chain length in fluid phosphatidylcholine vesicles. J. Mol. Biol. 166, 211–217 (1983).
Gruner, S.M. Intrinsic curvature hypothesis for biomembrane lipid composition: a role for nonbilayer lipids. Proc. Natl. Acad. Sci. USA 82, 3665–3669 (1985).
Martinac, B., Adler, J. & Kung, C. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature 348, 261–263 (1990).
Lundbaek, J.A. & Andersen, O.S. Lysophospholipids modulate channel function by altering the mechanical properties of lipid bilayers. J. Gen. Physiol. 104, 645–673 (1994).
Casado, M. & Ascher, P. Opposite modulation of NMDA receptors by lysophospholipids and arachidonic acid: common features with mechanosensitivity. J. Physiol. 513, 317–330 (1998).
Maingret, F., Patel, A.J., Lesage, F., Lazdunski, M. & Honoré, E. Lysophospholipids open the two-pore domain mechano-gated K+ channels TREK-1 and TRAAK. J. Biol. Chem. 275, 10128–10133 (2000).
Mandersloot, J.G., Reman, F.C., Van Deenen, L.L. & De Gier, J. Barrier properties of lecithin/lysolecithin mixtures. Biochim. Biophys. Acta 382, 22–26 (1975).
Hubbell, W.L., Gross, A., Langen, R. & Lietzow, M.A. Recent advances in site-directed spin labeling of proteins. Curr. Opin. Struct. Biol. 8, 649–656 (1998).
Hubbell, W.L., Cafiso, D.S. & Altenbach, C. Identifying conformational changes with site-directed spin labeling. Nature Struct. Biol. 7, 735–739 (2000).
Mchaourab, H. & Perozo, E. in Biological Magnetic Resonance Vol. 19 (ed. S. Eaton, G.E. & Berliner, L.) 155–218 (Kluwer-Plenum, New York; 2000).
Perozo, E., Kloda, A., Cortes, D.M. & Martinac, B. Site-directed spin-labeling analysis of reconstituted MscL in the closed state. J. Gen. Physiol. 118, 193–206 (2001).
Ou, X., Blount, P., Hoffman, R.J. & Kung, C. One face of a transmembrane helix is crucial in mechanosensitive channel gating. Proc. Natl. Acad. Sci. USA 95, 11471–11475 (1998).
Sukharev, S., Betanzos, M., Chiang, C.-S. & Guy, H.R. The gating mechanism of the large mechanosensitive channel MscL. Nature 409, 720–724 (2001).
Sukharev, S., Durell, S.R. & Guy, H.R. Structural models of the MscL gating mechanism. Biophys. J. 81, 917–936 (2001).
Gullingsrud, J., Kosztin, D. & Schulten, K. Structural determinants of MscL gating studied by molecular dynamics simulations. Biophys. J. 80, 2074–2081 (2001).
Sukharev, S.I., Sigurdson, W.J., Kung, C. & Sachs, F. Energetic and spatial parameters for gating of the bacterial large conductance mechanosensitive channel, MscL. J. Gen. Physiol. 113, 525–540 (1999).
Delcour, A.H., Martinac, B., Adler, J. & Kung, C. Modified reconstitution method used in patch-clamp studies of Escherichia coli ion channels. Biophys. J. 56, 631–636 (1989).
Häse, C.C., Le Dain, A.C. & Martinac, B. Purification and functional reconstitution of the recombinant large mechanosensitive ion channel (MscL) of Escherichia coli. J. Biol. Chem. 270, 18329–18334 (1995).
Cruickshank, C.C., Minchin, R.F., Le Dain, A.C. & Martinac, B. Estimation of the pore size of the large-conductance mechanosensitive ion channel of Escherichia coli. Biophys. J. 73, 1925–1931 (1997).
Cortes, D.M., Cuello, L.G. & Perozo, E. Molecular architecture of full-length KcsA: role of cytoplasmic domains in ion permeation and activation gating. J. Gen. Physiol. 117, 165–180 (2001).
Mchaourab, H.S., Lietzow, M.A., Hideg, K. & Hubbell, W.L. Motion of spin-labeled side chains in T4 lysozyme. Correlation with protein structure and dynamics. Biochemistry 35, 7692–7704 (1996).
Columbus, L., Kálai, T., Jekö, J., Hideg, K. & Hubbell, W.L. Molecular motion of spin labeled side chains in α-helices: analysis by variation of side chain structure. Biochemistry 40, 3828–3846 (2001).
Perozo, E., Cortes, D.M. & Cuello, L.G. Three-dimensional architecture and gating mechanism of a K+ channel studied by EPR spectroscopy. Nature Struct. Biol. 5, 459–469 (1998).
Acknowledgements
We thank T. Thompsom and R. Biltonen for insightful discussions and C. Ptak for critically reading the manuscript. Support and encouragement from S. Mochel is greatly appreciated. This work was supported in part by NIH (E.P.) and the McKnight endowment fund for neuroscience (E.P.), the Australian Research Council (B.M.) and the Australian Academy of Science (Scientific Visit Award to B.M).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Perozo, E., Kloda, A., Cortes, D. et al. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat Struct Mol Biol 9, 696–703 (2002). https://doi.org/10.1038/nsb827
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsb827