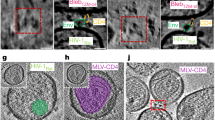
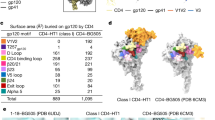
Abstract
Infection with HIV-1 is initiated by fusion of cellular and viral membranes. The gp41 subunit of the HIV-1 envelope plays a major role in this process, but the structure of gp41 is unknown. We have identified a stable, proteinase-resistant structure comprising two peptides, N-51 and C-43, derived from a recombinant protein fragment of the gp41 ectodomain. In isolation, N-51 is predominantly aggregated and C-43 is unfolded. When mixed, however, these peptides associate to form a stable, α-helical, discrete trimer of heterodimers. Proteolysis experiments indicate that the relative orientation of the N-51 and C-43 helices in the complex is antiparallel. We propose that N-51 forms an interior, parallel, homotrimeric, coiled-coil core, against which three C-43 helices pack in an antiparallel fashion. We suggest that this α-helical, trimeric complex is the core of the fusion-competent state of the HIV-1 envelope.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Bour, S., Geleziunas, R. & Wainberg, M.A. The human immunodeficiency virus type 1 (HIV-1) CD4 receptor and its central role in promotion of HIV-1 infection. Micriobiol. Rev. 59, 63–93 (1995).
Nara, P.L., Garrity, R.R. & Goudsmit, J. Neutralization of HIV-1: a paradox of humoral proportions. FASEB J. 5, 2437–2455 (1991).
Klatzmann, D. et al. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature 312, 767–768 (1984).
McDougal, J.S. et al. Binding of the HTLV-III/LAV to T4+ T cells by a complex of the 100 K viral protein and the T4 molecule. Science 231, 382–385 (1986).
Gallaher, W.R. Detection of a fusion peptide sequence in the transmembrane protein of human immunodeficiency virus. Cell 50, 327–328 (1987).
Gallaher, W.R., Ball, J.M., Garry, R.R., Griffin, M.C. & Montelaro, R.C. A general model for the transmembrane proteins of HIV and other retroviruses. AIDS Res. Hum. Retroviruses 5, 431–440 (1989).
White, J.M. Membrane fusion. Science 258, 917–924 (1992).
Stegmann, T., Delfino, J.M., Richards, F.M. & Helenius, A. The HA2 subunit of influenza hemagglutinin inserts into the target membrane prior to fusion. J. biol. Chem. 266, 18404–18410 (1991).
Tsurudome, M. et al. Lipid interactions of the hemagglutinin HA2 NH2-terminal segment during influenza virus-induced membrane fusion. J. biol. Chem. 267, 20225–20232 (1992).
Delwart, E.J., Mosialos, G. & Gilmore, T. Retroviral envelope glycoproteins contain a leucine zipper like repeat. AIDS Res. Hum. Retroviruses 6, 703–706 (1990).
Chambers, P., Pringle, C.R. & Easton, A.J. Heptad repeat sequences are located adjacent to hydrophobic regions in several types of virus fusion glycoproteins. J. Gen. Virol. 71, 3075–3080 (1990).
Hughson, F.M. Structural characterization of viral fusion proteins. Cur. Biol. 5, 265–274 (1995).
Moore, J.P., McKeating, J.A., Weiss, R.A. & Sattentau, Q.J. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science 250, 1139–1142 (1990).
Hart, T.K. et al. Binding of soluble CD4 proteins to human immunodeficiency virus type 1 and infected cells induces release of envelope glycoprotein gp120. Proc. natn. Acad. Sci. U.S.A. 88, 2189–2193 (1991).
Sattentau, Q.J. & Moore, J.P. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J. Exp. Med. 174, 407–415 (1991).
Allan, J.S. Receptor-mediated activation of immunodeficiency viruses in viral fusion. Science 252, 1322–1323 (1991).
Moore, J.P., McKeating, J.A., Weiss, R.A., Clapham, P.R. & Sattentau, Q.J. Receptor-mediated activation of immunodeficiency viruses in viral fusion. Science 252, 1322–1323 (1991).
Carr, C.M. & Kim, P.S. A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell 73, 823–832 (1993).
Sullivan, N., Sun, Y., Li, J., Hofmann, W. & Sodroski, J. Replicative function and neutralization sensitivity of envelope glycoproteins from primary and T-cell line-passaged human immunodeficiency virus type 1 isolates. J. Virol. 69, 4413–4422 (1985).
Lupas, A., VanDyke, M. & Stock, J. Predicting coiled coils from protein sequences. Science 252, 1162–1164 (1991).
Berger, B.A., Wilson, D.B., Wolf, E., Tonchev, T., Milla, M. & Kim, P.S. Predicting coiled coils using pairwise residue correlations. Proc. natn. Acad. Sci. U.S.A. 92, 8259–8263 (1995).
Jaenicke, R. & Rudolph, R. Refolding and association of oligomeric proteins. Meth. Enzymol. 131, 218–250 (1986).
Blacklow, S.C., Lu, M. & Kim, P.S. A trimeric subdomain of the simian immunodeficiency virus envelope glycoprotein. Biochemistry in the press.
Dubay, J.W., Roberts, S.J., Brody, B. & Hunter, E. Mutations in the leucine zipper of the human immunodeficiency virus type 1 transmembrane glycoprotein affect fusion and infectivity. J. Virol. 66, 4748–4756 (1992).
Chen, S.S., Lee, C.N., Lee, W.R., Mclntosh, K. & Lee, T.H. Mutational analysis of the leucine zipper-like motif of the human immunodeficiency virus type 1 envelope transmembrane glycoprotein. J. Virol. 67, 3615–3619 (1993).
Wild, C.T., Oas, T., McDanal, C.B., Bolognesi, D. & Matthews, T.J. A synthetic peptide inhibitor of human immunodeficiency virus replication: correlation between solution structure and viral inhibition. Proc. natn. Acad. Sci. U.S.A. 89, 10537–10541 (1992).
Jiang, S., Lin, K., Strick, N. & Neurath, A.R. HIV-1 inhibition by a peptide. Nature 365, 113 (1993).
Wild, C.T., Shugars, D.C., Greenwell, T.K., McDanal, C.B. & Matthews, T.J. Peptides corresponding to a predictive α-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc. natn. Acad. Sci. U.S.A. 91, 9770–9774 (1994).
Hodges, R.S., Sodek, J., Smillie, L.B. & Jurasek, L. Tropomyosin: amino acid sequence and coiled-coil structure. Cold Spring Harb. Symp. Quant. Biol. 37, 299–310 (1972).
McLachlan, A.D. & Stewart, M. Tropomyosin coiled-coil interactions: evidence for an unstaggered structure. J. molec. Biol. 98, 293–304 (1975).
O'Shea, E.K., Klemm, J.D., Kim, P.S. & Alber, T. X-ray structure of the GCN4 leucine zipper, a two-stranded, parallel, coiled coil. Science 254, 539–544(1991).
Wilson, I.A., Skehel, J.J. & Wiley, D.C. Structure of the hemagglutinin membrane glycoprotein of influenza virus at 3 Å resolution. Nature 289, 366–373 (1981).
Harbury, P.B., Zhang, T., Kim, P.S. & Alber, T. A switch between two-, three-, and four-stranded coiled coils in GCN4 leucine zipper mutants. Science 262, 1401–1407 (1993).
Harbury, P.B., Kim, P.S. & Alber, T. Crystal structure of an isoleucine-zipper trimer. Nature 371, 80–83 (1994).
Jiang, S., Lin, K., Strick, N. & Neurath, A.R. Inhibition of HIV-1 infection by a fusion domain binding peptide from the HIV-1 envelope glycoprotein gp41. Biochem. Biophys. Res. Commun. 195, 533–538 (1993).
Neurath, A.R., Lin, K., Strick, N. & Jiang, S. Two partially overlapping antiviral peptides from the external portion of HIV type 1 glycoprotein 41, adjoining the transmembrane region, affect the glycoprotein 41 fusion domain. AIDS Res. Hum. Retroviruses 11, 189–190 (1995).
Wild, C.T., Greenwell,T., Shugars, D., Rimsky-Clarke, L. & Matthews, T. The inhibitory activity of an HIV-1 type peptide correlates with its ability to interact with a leucine zipper structure. AIDS Res. Hum. Retroviruses 11, 323–325 (1995).
Herskowitz, I. Functional inactivation of genes by dominant negative mutations. Nature 329, 219–222 (1987).
Chen, C.H., Matthews, T.J., McDanal, C.B., Bolognesi, D.P. & Greenberg, M.L. A molecular clasp in the human immunodeficiency virus (HIV) type 1 TM protein determines the anti-HIV activity of gp41 derivatives: implication for viral fusion. J. Virol. 69, 3771–3777 (1995).
Gelderblom, H.R. et al. Fine structure of human immunodeficiency virus (HIV), immunolocalization of structural proteins and virus-cell relation. Micron. Microsc. Acta 19, 41–60 (1988).
Weiss, C.D., Levy, J.A. & White, J.M. Oligomeric organization on infectious human immunodeficiency virus type 1 particles. J. Virol. 64, 5674–5677 (1990).
Schawaller, M., Smith, G.E., Skehel, J.J. & Wiley, D.C. Studies with crosslinking reagents on the oligomeric structure of the env glycoprotein of HIV. Virology 172, 367–369 (1989).
Pinter, A. et al. Oligomeric structure of gp41, the transmembrane protein of human immunodeficiency virus type 1. J. Virol. 63, 267–279 (1989).
Earl, P.L., Doms, R.W. & Moss, B. Oligomeric structure of the human immunodeficiency virus type 1 envelope glycoprotein. Proc. natn. Acad. Sci. U.S.A. 87, 648–652 (1990).
Thomas, D.J. et al. gp160, the envelope glycoprotein of human immunodeficiency virus type 1, is a dimer of 125-kilodalton subunits stabilized through interactions between their gp41 domains. J. Virol. 65, 3797–3803 (1991).
Fass, D. & Kim, P.S. Dissection of a retrovirus envelope reveals structural similarity to influenza hemagglutinin. Curr. Biol. in the press.
Carr, C.M. & Kim, P.S. Flu virus invasion: halfway there. Science 266, 234–236 (1994).
Bullough, P.A., Hughson, F.M., Skehel, J.J. & Wiley, D.C. Structure of influenza hemagglutinin at the pH of membrane fusion. Nature 371, 37–43 (1994).
Doering, D. Functional and Structural Studies of a Small f-actin Binding Protein. PhD thesis, Massachusetts Institute of Technology, Cambridge, Massachusetts (1992).
Kunkel, T.A., Roberts, J.D. & Zakour, R.A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Meths. Enzymol. 154, 367–382 (1987).
Sambrook, J., Fritsch, E.F. & Maniatis, T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York (1989).
Peng, Z.Y. & Kim, P.S. A protein dissection study of a molten globule. Biochemistry 33, 2136–2141 (1994).
Edelhoch, H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry 6, 1948–1954 (1967).
Cantor, C. & Schimmel, P. Biophysical Chemistry, Part III. W.H. Freeman and Company, New York, 1131–1132 (1980).
O'Shea, E.K., Rutkowski, R. & Kim, P.S. Evidence that the leucine zipper is a coiled coil. Science 243, 538–542 (1989).
Laue, T.M., Shah, B.D., Ridgeway, T.M. & Pelletier, S.L. Computer-aided interpretation of analytical sedimentation data for proteins. In Analytical Ultracentrifugation in Biochemistry and Polymer Science (Harding, S.E., Rowe, A.J. & Horton, J.C eds) 90–125 (Royal Society of Chemistry, Cambridge, 1992).
Kozarsky, K. et al. Glycosylation and processing of the human immunodeficiency virus type 1 envelope protein. AIDS 2, 163–169 (1989).
Harada, S., Koyanagi, Y. & Yamamoto, N. Infection of HTLV-III/LAV in HTLV-1-carrying cells MT-2 and MT-4 and application in a plaque assay. Science 229, 563–566 (1985).
Schagger, H. & von Jagow, G. Tricine-sodium dodecylsulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochemistry 166, 368–379 (1987).
Allan, J.S., Strauss, J. & Buck, D.W. Enhancement of SIV infection with soluble receptor molecules. Science 247, 1084–1088 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lu, M., Blacklow, S. & Kim, P. A trimeric structural domain of the HIV-1 transmembrane glycoprotein. Nat Struct Mol Biol 2, 1075–1082 (1995). https://doi.org/10.1038/nsb1295-1075
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb1295-1075
This article is cited by
-
Identification of the natural product berberine as an antiviral drug
AMB Express (2020)
-
Entry Inhibitors: Efficient Means to Block Viral Infection
The Journal of Membrane Biology (2020)
-
Evaluation of the contribution of the transmembrane region to the ectodomain conformation of the human immunodeficiency virus (HIV-1) envelope glycoprotein
Virology Journal (2017)
-
The β20–β21 of gp120 is a regulatory switch for HIV-1 Env conformational transitions
Nature Communications (2017)
-
HIV-1 gp41-targeting fusion inhibitory peptides enhance the gp120-targeting protein-mediated inactivation of HIV-1 virions
Emerging Microbes & Infections (2017)