Abstract
Follicular helper T (TFH) cells are essential for B-cell maturation and immunoglobulin production after immunization with thymus-dependent antigens. Nevertheless, the development and function of TFH cells have been less clearly defined than classic CD4+ effector T-cell subsets, including T-helper-1 (TH1), TH2 and TH17 cells. As such, our understanding of the genesis of TFH cells in humans and their role in the development of autoimmunity remains incomplete. However, evidence from animal models of systemic lupus erythematosus (SLE) and patients with systemic autoimmune diseases suggests that these cells are necessary for pathogenic autoantibody production, in a manner analogous to their role in promotion of B-cell maturation during normal immune responses. In this Review, I discuss the findings that have increased our knowledge of TFH-cell development and function in normal and aberrant immune responses. Such information might improve our understanding of autoimmune diseases, such as SLE, and highlights the potential of TFH cells as therapeutic targets in these diseases.
Key Points
-
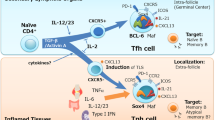
Follicular helper T (TFH) cells—a subset of CD4+ T cells—are located within the B-cell follicles of secondary lymphoid organs
-
TFH cells are important regulators of B-cell maturation within germinal centres during normal immune responses
-
Characterization of the developmental program of TFH cells could aid their identification and provide insight into the function of these cells in normal and autoimmune responses
-
The transcription factor B-cell lymphoma 6 is both necessary and sufficient for development of TFH cells, controlling expression of molecules essential for TFH-cell trafficking and function
-
TFH cells promote pathogenic autoantibody production in systemic autoimmunity, and potentially represent novel therapeutic targets in autoimmune diseases
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Campbell, D. J., Kim, C. H. & Butcher, E. C. Separable effector T cell populations specialized for B cell help or tissue inflammation. Nat. Immunol. 2, 876–881 (2001).
Murphy, K. M. & Reiner, S. L. The lineage decisions of helper T cells. Nat. Rev. Immunol. 2, 933–944 (2002).
Szabo, S. J. et al. A novel transcription factor, T-bet, directs TH1 lineage commitment. Cell 100, 655–669 (2000).
Zheng, W. & Flavell, R. A. The transcription factor GATA-3 is necessary and sufficient for TH2 cytokine gene expression in CD4 T cells. Cell 89, 587–596 (1997).
Dang, E. V. et al. Control of T(H)17/T(REG) balance by hypoxia-inducible factor 1. Cell 146, 772–784 (2011).
Ivanov, I. I. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T Helper Cells. Cell 126, 1121–1133 (2006).
Yang, X. O. et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors RORα and RORγ. Immunity 28, 29–39 (2008).
Liang, S. C. et al. Interleukin (IL)-22 and IL-17 are coexpressed by TH17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203, 2271–2279 (2006).
Eyerich, S. et al. TH22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J. Clin. Invest. 119, 3573–3585 (2009).
Langrish, C. L. et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201, 233–240 (2005).
Koenders, M. I. et al. Blocking of interleukin-17 during reactivation of experimental arthritis prevents joint inflammation and bone erosion by decreasing RANKL and interleukin-1. Am. J. Pathol. 167, 141–149 (2005).
Crispín, J. C. & Tsokos, G. C. Interleukin-17-producing T cells in lupus. Curr. Opin. Rheum. 22, 499–503 (2010).
Parker, D. C. T cell-dependent B cell activation. Annu. Rev. Immunol. 11, 331–360 (1993).
Liu, Y. J., Zhang, J., Lane, P. J., Chan, E. Y. & MacLennan, I. C. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur. J. Immunol. 21, 2951–2962 (1991).
MacLennan, I. C. et al. Extrafollicular antibody responses. Immunol. Rev. 194, 8–18 (2003).
Jacob, J., Kassir, R. & Kelsoe, G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J. Exp. Med. 173, 1165–1175 (1991).
Berek, C., Berger, A. & Apel, M. Maturation of the immune response in germinal centers. Cell 67, 1121–1129 (1991).
Coffey, F., Alabyev, B. & Manser, T. Initial clonal expansion of germinal center B cells takes place at the perimeter of follicles. Immunity 30, 599–609 (2009).
Kerfoot, S. M. et al. Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone. Immunity 34, 947–960 (2011).
Choi, Y. S. et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity 34, 932–946 (2011).
Willimann, K. et al. The chemokine SLC is expressed in T cell areas of lymph nodes and mucosal lymphoid tissues and attracts activated T cells via CCR7. Eur. J. Immunol. 28, 2025–2034 (1998).
Nagira, M. et al. A lymphocyte-specific CC chemokine, secondary lymphoid tissue chemokine (SLC), is a highly efficient chemoattractant for B cells and activated T cells. Eur. J. Immunol. 28, 1516–1523 (1998).
Luther, S. A. et al. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J. Immunol. 169, 424–433 (2002).
Haynes, N. M. et al. Role of CXCR5 and CCR7 in follicular TH cell positioning and appearance of a programmed cell death gene-1High germinal center-associated subpopulation. J. Immunol. 179, 5099–5108 (2007).
Poholek, A. C. et al. In vivo regulation of Bcl6 and T follicular helper cell development. J. Immunol. 185, 313–326 (2010).
Veerman, K. M. et al. Interaction of the selectin ligand PSGL-1 with chemokines CCL21 and CCL19 facilitates efficient homing of T cells to secondary lymphoid organs. Nat. Immunol. 8, 532–539 (2007).
Ansel, K. M., McHeyzer-Williams, L. J., Ngo, V. N., McHeyzer-Williams, M. G. & Cyster, J. G. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J. Exp. Med. 190, 1123–1134 (1999).
Walker, L. S. et al. Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXC chemokine receptor 5-positive CD4 cells and germinal centers. J. Exp. Med. 190, 1115–1122 (1999).
Gunn, M. D. et al. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature 391, 799–803 (1998).
Hardtke, S., Ohl, L. & Forster, R. Balanced expression of CXCR5 and CCR7 on follicular T helper cells determines their transient positioning to lymph node follicles and is essential for efficient B-cell help. Blood 106, 1924–1931 (2005).
Akiba, H. et al. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J. Immunol. 175, 2340–2348 (2005).
Nurieva, R. I. et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 29, 138–149 (2008).
Johnston, R. J. et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325, 1006–1010 (2009).
Baumjohann, D., Okada, T. & Ansel, K. M. Cutting edge: distinct waves of BCL6 expression during T follicular helper cell development. J. Immunol. 187, 2089–2092 (2011).
Dong, C., Temann, U. A. & Flavell, R. A. Cutting edge: critical role of inducible costimulator in germinal center reactions. J. Immunol. 166, 3659–3662 (2001).
Odegard, J. M. et al. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J. Exp. Med. 205, 2873–2886 (2008).
Bossaller, L. et al. ICOS deficiency is associated with a severe reduction of CXCR5+ CD4 germinal center TH cells. J. Immunol. 177, 4927–4932 (2006).
Burmeister, Y. et al. ICOS controls the pool size of effector-memory and regulatory T cells. J. Immunol. 180, 774–782 (2008).
Good-Jacobson, K. L. et al. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat. Immunol. 11, 535–542 (2010).
Reif, K. et al. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature 416, 94–99 (2002).
Hannedouche, S. et al. Oxysterols direct immune cell migration via EBI2. Nature 475, 524–527 (2011).
Liu, C. et al. Oxysterols direct B-cell migration through EBI2. Nature 475, 519–523 (2011).
Pereira, J. P., Kelly, L. M., Xu, Y. & Cyster, J. G. EBI2 mediates B cell segregation between the outer and centre follicle. Nature 460, 1122–1126 (2009).
Gatto, D., Paus, D., Basten, A., Mackay, C. R. & Brink, R. Guidance of B Cells by the orphan G protein-coupled receptor EBI2 shapes humoral immune responses. Immunity 31, 259–269 (2009).
Crotty, S., Kersh, E. N., Cannons, J., Schwartzberg, P. L. & Ahmed, R. SAP is required for generating long-term humoral immunity. Nature 421, 282–287 (2003).
Cannons, J. L. et al. SAP regulates T cell-mediated help for humoral immunity by a mechanism distinct from cytokine regulation. J. Exp. Med. 203, 1551–1565 (2006).
Qi, H., Cannons, J. L., Klauschen, F., Schwartzberg, P. L. & Germain, R. N. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature 455, 764–769 (2008).
Cunningham, A. F., Serre, K., Mohr, E., Khan, M. & Toellner, K. M. Loss of CD154 impairs the TH2 extrafollicular plasma cell response but not early T cell proliferation and interleukin-4 induction. Immunology 113, 187–193 (2004).
Deenick, E. K. et al. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity 33, 241–253, (2010).
Schaerli, P., Loetscher, P. & Moser, B. Cutting edge: induction of follicular homing precedes effector TH cell development. J. Immunol. 167, 6082–6086 (2001).
Springer, T. A. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu. Rev. Physiol. 57, 827–872 (1995).
Austrup, F. et al. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflammed tissues. Nature 385, 81–83 (1997).
Ley, K. & Kansas, G. S. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat. Rev. Immunol. 4, 325–335 (2004).
Kim, C. H. et al. Rules of chemokine receptor association with T cell polarization in vivo. J. Clin. Invest. 108, 1331–1339 (2001).
Dent, A. L., Shaffer, A. L., Yu, X., Allman, D. & Staudt, L. M. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science 276, 589–592 (1997).
Ye, B. H. et al. The BCL-6 proto-oncogene controls germinal-centre formation and TH2-type inflammation. Nat. Genet. 16, 161–170 (1997).
Chtanova, T. et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-TH1/TH2 effector cells that provide help for B cells. J. Immunol. 173, 68–78 (2004).
Nurieva, R. I. et al. Bcl6 mediates the development of T follicular helper cells. Science 325, 1001–1005 (2009).
Yu, D. et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 31, 457–468 (2009).
Johnston, R. J., Choi, Y. S., Diamond, J. A., Yang, J. A. & Crotty, S. STAT5 is a potent negative regulator of TFH cell differentiation. J. Exp. Med. http://dx.doi.org/10.1084/jem.20111174.
Kitano, M. et al. Bcl6 protein expression shapes pre-germinal center B cell dynamics and follicular helper T cell heterogeneity. Immunity 34, 961–972 (2011).
Garside, P. et al. Visualization of specific B and T lymphocyte interactions in the lymph node. Science 281, 96–99 (1998).
Breitfeld, D. et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 192, 1545–1552 (2000).
Schaerli, P. et al. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 192, 1553–1562 (2000).
Kim, C. H. et al. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J. Exp. Med. 193, 1373–1381 (2001).
Jacob, J., Kelsoe, G., Rajewsky, K. & Weiss, U. Intraclonal generation of antibody mutants in germinal centres. Nature 354, 389–392 (1991).
Liu, Y. J. et al. Within germinal centers, isotype switching of immunoglobulin genes occurs after the onset of somatic mutation. Immunity 4, 241–250 (1996).
Parrish-Novak, J. et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 408, 57–63 (2000).
Mehta, D. S. et al. IL-21 induces the apoptosis of resting and activated primary B cells. J. Immunol. 170, 4111–4118 (2003).
Jin, H., Carrio, R., Yu, A. & Malek, T. R. Distinct activation signals determine whether IL-21 induces B cell costimulation, growth arrest, or Bim-dependent apoptosis. J. Immunol. 173, 657–665 (2004).
Ozaki, K. et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J. Immunol. 173, 5361–5371 (2004).
Ettinger, R. et al. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J. Immunol. 175, 7867–7879 (2005).
Kuchen, S. et al. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T cell-B cell collaboration. J. Immunol. 179, 5886–5896 (2007).
Linterman, M. A. et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J. Exp. Med. 207, 353–363 (2010).
Zotos, D. et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J. Exp. Med. 207, 365–378 (2010).
Eto, D. et al. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (TFH) differentiation. PLoS One 6, e17739 (2011).
Ozaki, K. et al. A critical role for IL-21 in regulating immunoglobulin production. Science 298, 1630–1634 (2002).
Hsu, H. C. et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat. Immunol. 9, 166–175 (2008).
Reinhardt, R. L., Liang, H. E. & Locksley, R. M. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat. Immunol. 10, 385–393 (2009).
King, I. L. & Mohrs, M. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. J. Exp. Med. 206, 1001–1007 (2009).
Bauquet, A. T. et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat. Immunol. 10, 167–175 (2009).
Hams, E. et al. Blockade of B7-H1 (programmed death ligand 1) enhances humoral immunity by positively regulating the generation of T follicular helper cells. J. Immunol. 186, 5648–5655 (2011).
Mempel, T. R., Henrickson, S. E. & Von Andrian, U. H. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427, 154–159 (2004).
Callard, R. E., Armitage, R. J., Fanslow, W. C. & Spriggs, M. K. CD40 ligand and its role in X-linked hyper-IgM syndrome. Immunol. Today 14, 559–564 (1993).
Xu, J. et al. Mice deficient for the CD40 ligand. Immunity 1, 423–431 (1994).
McAdam, A. J. et al. ICOS is critical for CD40-mediated antibody class switching. Nature 409, 102–105 (2001).
Lee, S. K. et al. B cell priming for extrafollicular antibody responses requires Bcl-6 expression by T cells. J. Exp. Med. 208, 1377–1388 (2011).
Barral, P. et al. B cell receptor-mediated uptake of CD1d-restricted antigen augments antibody responses by recruiting invariant NKT cell help in vivo. Proc. Natl Acad. Sci. USA 105, 8345–8350 (2008).
Leadbetter, E. A. et al. NK T cells provide lipid antigen-specific cognate help for B cells. Proc. Natl Acad. Sci. USA 105, 8339–8344 (2008).
King, I. L. et al. Invariant natural killer T cells direct B cell responses to cognate lipid antigen in an IL-21-dependent manner. Nat. Immunol. 13, 44–50, (2011).
Chang, P. P. et al. Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat. Immunol. 13, 35–43 (2011).
Vinuesa, C. G., Sanz, I. & Cook, M. C. Dysregulation of germinal centres in autoimmune disease. Nat. Rev. Immunol. 9, 845–857 (2009).
Linterman, M. A. et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat. Med. 17, 975–982 (2011).
Wollenberg, I. et al. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J. Immunol. 187, 4553–4560 (2011).
Chung, Y. et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat. Med. 17, 983–988 (2011).
Alexander, C. M. et al. T regulatory cells participate in the control of germinal centre reactions. Immunology 133, 452–468 (2011).
Jang, E. et al. Foxp3+ regulatory T cells control humoral autoimmunity by suppressing the development of long-lived plasma cells. J. Immunol. 186, 1546–1553 (2011).
Ritchie, A. W., James, K. & Micklem, H. S. The distribution and possible significance of cells identified in human lymphoid tissue by the monoclonal antibody HNK-1. Clin. Exp. Immunol. 51, 439–447 (1983).
Banerjee, D. & Thibert, R. F. Natural killer-like cells found in B-cell compartments of human lymphoid tissues. Nature 304, 270–272 (1983).
Kim, J. R., Lim, H. W., Kang, S. G., Hillsamer, P. & Kim, C. H. Human CD57+ germinal center-T cells are the major helpers for GC-B cells and induce class switch recombination. BMC Immunol. 6, 3 (2005).
Casamayor-Palleja, M., Khan, M. & MacLennan, I. C. A subset of CD4+ memory T cells contains preformed CD40 ligand that is rapidly but transiently expressed on their surface after activation through the T cell receptor complex. J. Exp. Med. 181, 1293–1301 (1995).
Rasheed, A. U., Rahn, H. P., Sallusto, F., Lipp, M. & Müller, G. Follicular B helper T cell activity is confined to CXCR5hiICOShi CD4 T cells and is independent of CD57 expression. Eur. J. Immunol. 36, 1892–1903 (2006).
Lim, H. W. & Kim, C. H. Loss of IL-7 receptor α on CD4+ T cells defines terminally differentiated B cell-helping effector T cells in a B cell-rich lymphoid tissue. J. Immunol. 179, 7448–7456 (2007).
Chevalier, N. et al. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J. Immunol. 186, 5556–5568 (2011).
Morita, R. et al. Human blood CXCR5+CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 34, 108–121 (2011).
MacLeod, M. K. et al. Memory CD4 T cells that express CXCR5 provide accelerated help to B cells. J. Immunol. 186, 2889–2896 (2011).
Bentebibel, S. E., Schmitt, N., Banchereau, J. & Ueno, H. Human tonsil B-cell lymphoma 6 (BCL6)-expressing CD4+ T-cell subset specialized for B-cell help outside germinal centers. Proc. Natl Acad. Sci. USA 108, E488–E497 (2011).
Dogan, I. et al. Multiple layers of B cell memory with different effector functions. Nat. Immunol. 10, 1292–1299 (2009).
Pepper, M., Pagán, A. J., Igyártó, B. Z., Taylor, J. J. & Jenkins, M. K. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity 35, 583–595 (2011).
Marshall, H. D. et al. Differential expression of Ly6C and T-bet distinguish effector and memory TH1 CD4+ cell properties during viral infection. Immunity 35, 633–646 (2011).
Simpson, N. et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 62, 234–244 (2010).
Vinuesa, C. G. et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature 435, 452–458 (2005).
Luzina, I. G. et al. Spontaneous formation of germinal centers in autoimmune mice. J. Leukoc. Biol. 70, 578–584 (2001).
Linterman, M. A. et al. Follicular helper T cells are required for systemic autoimmunity. J. Exp. Med. 206, 561–576 (2009).
Bertossi, A. et al. Loss of Roquin induces early death and immune deregulation but not autoimmunity. J. Exp. Med. 208, 1749–1756 (2011).
Pisitkun, P. et al. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science 312, 1669–1672 (2006).
Bubier, J. A. et al. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc. Natl Acad. Sci. USA 106, 1518–1523 (2009).
Herber, D. et al. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. J. Immunol. 178, 3822–3830 (2007).
Peng, S. L., Moslehi, J. & Craft, J. Roles of interferon-γ and interleukin-4 in murine lupus. J. Clin. Invest. 99, 1936–1946 (1997).
Masutani, K. et al. Predominance of TH1 immune response in diffuse proliferative lupus nephritis. Arthritis Rheum. 44, 2097–2106 (2001).
Mohan, C., Shi, Y., Laman, J. D. & Datta, S. K. Interaction between CD40 and its ligand gp39 in the development of murine lupus nephritis. J. Immunol. 154, 1470–1480 (1995).
Ma, J. et al. Autoimmune lpr/lpr mice deficient in CD40 ligand: spontaneous Ig class switching with dichotomy of autoantibody responses. J. Immunol. 157, 417–426 (1996).
Daikh, D. I., Finck, B. K., Linsley, P. S., Hollenbaugh, D. & Wofsy, D. Long-term inhibition of murine lupus by brief simultaneous blockade of the B7/CD28 and CD40/gp39 costimulation pathways. J. Immunol. 159, 3104–3108 (1997).
Grammer, A. C. et al. Abnormal germinal center reactions in systemic lupus erythematosus demonstrated by blockade of CD154–CD40 interactions. J. Clin. Invest. 112, 1506–1520 (2003).
Duffau, P. et al. Platelet CD154 potentiates interferon-α secretion by plasmacytoid dendritic cells in systemic lupus erythematosus. Sci. Transl. Med. 2, 47ra63 (2010).
Peters, A. L., Stunz, L. L. & Bishop, G. A. CD40 and autoimmunity: the dark side of a great activator. Semin. Immunol. 21, 293–300 (2009).
Iwai, H. et al. Involvement of inducible costimulator-B7 homologous protein costimulatory pathway in murine lupus nephritis. J. Immunol. 171, 2848–2854 (2003).
William, J., Euler, C., Christensen, S. & Shlomchik, M. J. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science 297, 2066–2070 (2002).
Hoyer, B. F. et al. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J. Exp. Med. 199, 1577–1584 (2004).
Rankin, A. L. et al. IL-21 receptor is required for the systemic accumulation of activated B and T lymphocytes in MRL/MpJ-Faslpr/lpr/J mice. J. Immunol. http://dx.doi.org/10.4049/jimmunol.1003871.
Hargreaves, D. C. et al. A coordinated change in chemokine responsiveness guides plasma cell movements. J. Exp. Med. 194, 45–56 (2001).
Cappione, A. 3rd. et al. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J. Clin. Invest. 115, 3205–3216 (2005).
Yang, J. H. et al. Expression and function of inducible costimulator on peripheral blood T cells in patients with systemic lupus erythematosus. Rheumatology (Oxford) 44, 1245–1254 (2005).
Chang, A. et al. In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J. Immunol. 186, 1849–1860 (2011).
Weyand, C. M., Kang, Y. M., Kurtin, P. J. & Goronzy, J. J. The power of the third dimension: tissue architecture and autoimmunity in rheumatoid arthritis. Curr. Opin. Rheumatol. 15, 259–266 (2003).
Cantaert, T. et al. B lymphocyte autoimmunity in rheumatoid synovitis is independent of ectopic lymphoid neogenesis. J. Immunol. 181, 785–794 (2008).
Victoratos, P. & Kollias, G. Induction of autoantibody-mediated spontaneous arthritis critically depends on follicular dendritic cells. Immunity 30, 130–142 (2009).
Acknowledgements
This work was partially supported by NIH grants R01 AR40072, R01 AR44076 and P30 AR053495, and by Rheuminations, Inc. and the Alliance for Lupus Research. The author also acknowledges the many helpful discussions at lab meetings and other forums with his past and present trainees.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Craft, J. Follicular helper T cells in immunity and systemic autoimmunity. Nat Rev Rheumatol 8, 337–347 (2012). https://doi.org/10.1038/nrrheum.2012.58
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2012.58