Abstract
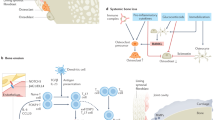
Rheumatoid arthritis (RA) is characterized by hyperplastic synovial pannus tissue, which mediates destruction of cartilage and bone. Fibroblast-like synoviocytes (FLS) are a key component of this invasive synovium and have a major role in the initiation and perpetuation of destructive joint inflammation. The pathogenic potential of FLS in RA stems from their ability to express immunomodulating cytokines and mediators as well as a wide array of adhesion molecule and matrix-modelling enzymes. FLS can be viewed as 'passive responders' to the immunoreactive process in RA, their activated phenotype reflecting the proinflammatory milieu. However, FLS from patients with RA also display unique aggressive features that are autonomous and vertically transmitted, and these cells can behave as primary promoters of inflammation. The molecular bases of this 'imprinted aggressor' phenotype are being clarified through genetic and epigenetic studies. The dual behaviour of FLS in RA suggests that FLS-directed therapies could become a complementary approach to immune-directed therapies in this disease. Pathophysiological characteristics of FLS in RA, as well as progress in targeting these cells, are reviewed in this manuscript.
Key Points
-
Fibroblast-like synoviocytes (FLS), normally found in the synovial intimal lining of diarthrodial joints, display an aggressive, invasive phenotype in rheumatoid arthritis (RA) and participate in joint destruction
-
FLS from patients with RA promote inflammatory cell recruitment and activation, pannus angiogenesis, cartilage degradation, and bone erosion
-
The phenotype of FLS from patients with RA is partly a passive response to the inflammatory milieu in vivo, and partly an imprinted feature that persists when the cells are cultured in vitro
-
Imprinted anomalies of FLS in RA arise, at least in part, through epigenetic modifications of the genome, such as altered microRNA expression and DNA methylation
-
Increased knowledge of the biology of FLS in RA will pave the way to novel FLS-targeted therapies with limited immunosuppressive action
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Firestein, G. S. Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum. 39, 1781–1790 (1996).
Müller-Ladner, U. et al. Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. Am. J. Pathol. 149, 1607–1615 (1996).
Lefèvre, S. et al. Synovial fibroblasts spread rheumatoid arthritis to unaffected joints. Nat. Med. 15, 1414–1420 (2009).
Bartok, B. & Firestein, G. S. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol. Rev. 233, 233–255 (2010).
Edwards, J. C., Leigh, R. D. & Cambridge, G. Expression of molecules involved in B lymphocyte survival and differentiation by synovial fibroblasts. Clin. Exp. Immunol. 108, 407–414 (1997).
Okazaki, M. et al. Molecular cloning and characterization of OB-cadherin, a new member of cadherin family expressed in osteoblasts. J. Biol. Chem. 269, 12092–12098 (1994).
Lee, D. M. et al. Cadherin-11 in synovial lining formation and pathology in arthritis. Science 315, 1006–1010 (2007).
Valencia, X. et al. Cadherin-11 provides specific cellular adhesion between fibroblast-like synoviocytes. J. Exp. Med. 200, 1673–1679 (2004).
Chang, S. K. et al. Cadherin-11 regulates fibroblast inflammation. Proc. Natl Acad. Sci. USA 108, 8402–8407 (2011).
Firestein, G. S., Yeo, M. & Zvaifler, N. J. Apoptosis in rheumatoid arthritis synovium. J. Clin. Invest. 96, 1631–1638 (1995).
Imamura, F. et al. Monoclonal expansion of synoviocytes in rheumatoid arthritis. Arthritis Rheum. 41, 1979–1986 (1998).
Matsumoto, S., Müller-Ladner, U., Gay, R. E., Nishioka, K. & Gay, S. Ultrastructural demonstration of apoptosis, Fas and Bcl-2 expression of rheumatoid synovial fibroblasts. J. Rheumatol. 23, 1345–1352 (1996).
Meinecke, I. et al. Modification of nuclear PML protein by SUMO-1 regulates Fas-induced apoptosis in rheumatoid arthritis synovial fibroblasts. Proc. Natl Acad. Sci. USA 104, 5073–5078 (2007).
Kumkumian, G. K. et al. Platelet-derived growth factor and IL-1 interactions in rheumatoid arthritis. Regulation of synoviocyte proliferation, prostaglandin production, and collagenase transcription. J. Immunol. 143, 833–837 (1989).
Lotz, M. & Guerne, P. A. Interleukin-6 induces the synthesis of tissue inhibitor of metalloproteinases-1/erythroid potentiating activity (TIMP-1/EPA). J. Biol. Chem. 266, 2017–2020 (1991).
Shigeyama, Y. et al. Expression of osteoclast differentiation factor in rheumatoid arthritis. Arthritis Rheum. 43, 2523–2530 (2000).
Pap, T., Aupperle, K. R., Gay, S., Firestein, G. S. & Gay, R. E. Invasiveness of synovial fibroblasts is regulated by p53 in the SCID mouse in vivo model of cartilage invasion. Arthritis Rheum. 44, 676–681 (2001).
Guerne, P. A., Zuraw, B. L., Vaughan, J. H., Carson, D. A. & Lotz, M. Synovium as a source of interleukin 6 in vitro. Contribution to local and systemic manifestations of arthritis. J. Clin. Invest. 83, 585–592 (1989).
Crow, M. K. Type I interferon in organ-targeted autoimmune and inflammatory diseases. Arthritis Res. Ther. 12 (Suppl. 1), S5 (2010).
Palmer, C. D., Mutch, B. E., Page, T. H., Horwood, N. J. & Foxwell, B. M. Bmx regulates LPS-induced IL-6 and VEGF production via mRNA stability in rheumatoid synovial fibroblasts. Biochem. Biophys. Res. Commun. 370, 599–602 (2008).
Nikitopoulou, I. et al. Autotaxin expression from synovial fibroblasts is essential for the pathogenesis of modeled arthritis. J. Exp. Med. 209, 925–933 (2012).
Stanczyk, J., Ospelt, C., Gay, R. E. & Gay, S. Synovial cell activation. Curr. Opin. Rheumatol. 18, 262–267 (2006).
Johnson, G. L. & Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298, 1911–1912 (2002).
Han, Z. et al. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J. Clin. Invest. 108, 73–81 (2001).
Yoshizawa, T. et al. Role of MAPK kinase 6 in arthritis: distinct mechanism of action in inflammation and cytokine expression. J. Immunol. 183, 1360–1367 (2009).
Korb, A. et al. Differential tissue expression and activation of p38 MAPK α, β, γ, and δ isoforms in rheumatoid arthritis. Arthritis Rheum. 54, 2745–2756 (2006).
Westra, J., Limburg, P. C., de Boer, P. & van Rijswijk, M. H. Effects of RWJ 67657, a p38 mitogen activated protein kinase (MAPK) inhibitor, on the production of inflammatory mediators by rheumatoid synovial fibroblasts. Ann. Rheum. Dis. 63, 1453–1459 (2004).
Inoue, T., Hammaker, D., Boyle, D. L. & Firestein, G. S. Regulation of p38 MAPK by MAPK kinases 3 and 6 in fibroblast-like synoviocytes. J. Immunol. 174, 4301–4306 (2005).
Guma, M. et al. Pro- and anti-inflammatory functions of the p38 pathway in rheumatoid arthritis: advantages of targeting upstream kinases MKK3 or MKK6. Arthritis Rheum. 64, 2887–2895 (2012).
Cohen, S. B. et al. Evaluation of the efficacy and safety of pamapimod, a p38 MAP kinase inhibitor, in a double-blind, methotrexate-controlled study of patients with active rheumatoid arthritis. Arthritis Rheum. 60, 335–344 (2009).
Genovese, M. C. et al. A 24-week, randomized, double-blind, placebo-controlled, parallel group study of the efficacy of oral SCIO-469, a p38 mitogen-activated protein kinase inhibitor, in patients with active rheumatoid arthritis. J. Rheumatol. 38, 846–854 (2011).
Shahrara, S., Castro-Rueda, H. P., Haines, G. K. & Koch, A. E. Differential expression of the FAK family kinases in rheumatoid arthritis and osteoarthritis synovial tissues. Arthritis Res. Ther. 9, R112 (2007).
Pap, T. et al. Cooperation of Ras- and c-Myc-dependent pathways in regulating the growth and invasiveness of synovial fibroblasts in rheumatoid arthritis. Arthritis Rheum. 50, 2794–2802 (2004).
Weisbart, R. H. et al. BRAF drives synovial fibroblast transformation in rheumatoid arthritis. J. Biol. Chem. 285, 34299–34303 (2010).
Abreu, J. R. et al. The Ras guanine nucleotide exchange factor RasGRF1 promotes matrix metalloproteinase-3 production in rheumatoid arthritis synovial tissue. Arthritis Res. Ther. 11, R121 (2009).
Han, Z. et al. Jun N-terminal kinase in rheumatoid arthritis. J. Pharmacol. Exp. Ther. 291, 124–130 (1999).
Svensson, C. I. et al. Gadd45β deficiency in rheumatoid arthritis: enhanced synovitis through JNK signaling. Arthritis Rheum. 60, 3229–3240 (2009).
Luo, Y. et al. Suppression of collagen-induced arthritis in growth arrest and DNA damage-inducible protein 45β-deficient mice. Arthritis Rheum. 63, 2949–2955 (2011).
Aupperle, K. et al. NF-κB regulation by IκB kinase-2 in rheumatoid arthritis synoviocytes. J. Immunol. 166, 2705–2711 (2001).
Oeckinghaus, A. & Ghosh, S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 1, a000034 (2009).
Okazaki, Y. et al. Effect of nuclear factor-κB inhibition on rheumatoid fibroblast-like synoviocytes and collagen induced arthritis. J. Rheumatol. 32, 1440–1447 (2005).
Zhang, H. G. et al. Gene therapy that inhibits nuclear translocation of nuclear factor κB results in tumor necrosis factor α-induced apoptosis of human synovial fibroblasts. Arthritis Rheum. 43, 1094–1105 (2000).
Pap, T. et al. Activation of synovial fibroblasts in rheumatoid arthritis: lack of expression of the tumour suppressor PTEN at sites of invasive growth and destruction. Arthritis Res. 2, 59–64 (2000).
Bartok, B. et al. PI3 kinase δ is a key regulator of synoviocyte function in rheumatoid arthritis. Am. J. Pathol. 180, 1906–1916 (2012).
Sweeney, S. E., Hammaker, D., Boyle, D. L. & Firestein, G. S. Regulation of c-Jun phosphorylation by the IκB kinase-ε complex in fibroblast-like synoviocytes. J. Immunol. 174, 6424–6430 (2005).
Sweeney, S. E., Corr, M. & Kimbler, T. B. Role of interferon regulatory factor 7 in serum-transfer arthritis: regulation of interferon-β production. Arthritis Rheum. 64, 1046–1056 (2012).
Korb, A., Pavenstadt, H. & Pap, T. Cell death in rheumatoid arthritis. Apoptosis 14, 447–454 (2009).
Wakisaka, S. et al. Modulation by proinflammatory cytokines of Fas/Fas ligand-mediated apoptotic cell death of synovial cells in patients with rheumatoid arthritis (RA). Clin. Exp. Immunol. 114, 119–128 (1998).
Audo, R. et al. Mechanisms and clinical relevance of TRAIL-triggered responses in the synovial fibroblasts of patients with rheumatoid arthritis. Arthritis Rheum. 63, 904–913 (2011).
Han, Z., Boyle, D. L., Shi, Y., Green, D. R. & Firestein, G. S. Dominant-negative p53 mutations in rheumatoid arthritis. Arthritis Rheum. 42, 1088–1092 (1999).
Firestein, G. S. et al. Apoptosis in rheumatoid arthritis: p53 overexpression in rheumatoid arthritis synovium. Am. J. Pathol. 149, 2143–2151 (1996).
Cha, H. S., Rosengren, S., Boyle, D. L. & Firestein, G. S. PUMA regulation and proapoptotic effects in fibroblast-like synoviocytes. Arthritis Rheum. 54, 587–592 (2006).
Schedel, J. et al. FLICE-inhibitory protein expression in synovial fibroblasts and at sites of cartilage and bone erosion in rheumatoid arthritis. Arthritis Rheum. 46, 1512–1518 (2002).
Franz, J. K. et al. Expression of sentrin, a novel antiapoptotic molecule, at sites of synovial invasion in rheumatoid arthritis. Arthritis Rheum. 43, 599–607 (2000).
Maciejewska-Rodrigues, H. et al. Epigenetics and rheumatoid arthritis: the role of SENP1 in the regulation of MMP-1 expression. J. Autoimmun. 35, 15–22 (2010).
Fassbender, H. G. & Simmling-Annefeld, M. The potential aggressiveness of synovial tissue in rheumatoid arthritis. J. Pathol. 139, 399–406 (1983).
Yamanishi, Y. et al. p53 tumor suppressor gene mutations in fibroblast-like synoviocytes from erosion synovium and non-erosion synovium in rheumatoid arthritis. Arthritis Res. Ther. 7, R12–R18 (2005).
Da Sylva, T. R., Connor, A., Mburu, Y., Keystone, E. & Wu, G. E. Somatic mutations in the mitochondria of rheumatoid arthritis synoviocytes. Arthritis Res. Ther. 7, R844–R851 (2005).
Lee, S. H. et al. Microsatellite instability and suppressed DNA repair enzyme expression in rheumatoid arthritis. J. Immunol. 170, 2214–2220 (2003).
Tak, P. P., Zvaifler, N. J., Green, D. R. & Firestein, G. S. Rheumatoid arthritis and p53: how oxidative stress might alter the course of inflammatory diseases. Immunol. Today 21, 78–82 (2000).
Harty, L. C. et al. Mitochondrial mutagenesis correlates with the local inflammatory environment in arthritis. Ann. Rheum. Dis. 71, 582–588 (2012).
Karouzakis, E., Gay, R. E., Gay, S. & Neidhart, M. Epigenetic deregulation in rheumatoid arthritis. Adv. Exp. Med. Biol. 711, 137–149 (2011).
Kuchen, S. et al. The L1 retroelement-related p40 protein induces p38δ MAP kinase. Autoimmunity 37, 57–65 (2004).
Nakano, K., Whitaker, J. W., Boyle, D. L., Wang, W. & Firestein, G. S. DNA methylome signature in rheumatoid arthritis. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2012-201526.
Grabiec, A. M., Korchynskyi, O., Tak, P. P. & Reedquist, K. A. Histone deacetylase inhibitors suppress rheumatoid arthritis fibroblast-like synoviocyte and macrophage IL-6 production by accelerating mRNA decay. Ann. Rheum. Dis. 71, 424–431 (2012).
Nakamachi, Y. et al. MicroRNA-124a is a key regulator of proliferation and monocyte chemoattractant protein 1 secretion in fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Rheum. 60, 1294–1304 (2009).
Stanczyk, J. et al. Altered expression of microRNA-203 in rheumatoid arthritis synovial fibroblasts and its role in fibroblast activation. Arthritis Rheum. 63, 373–381 (2011).
Duroux-Richard, I., Jorgensen, C. & Apparailly, F. What do microRNAs mean for rheumatoid arthritis? Arthritis Rheum. 64, 11–20 (2012).
Karouzakis, E., Gay, R. E., Gay, S. & Neidhart, M. Increased recycling of polyamines is associated with global DNA hypomethylation in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 64, 1809–1817 (2012).
Ballestar, E. An introduction to epigenetics. Adv. Exp. Med. Biol. 711, 1–11 (2011).
Bogdanos, D. P. et al. Twin studies in autoimmune disease: genetics, gender and environment. J. Autoimmun. 38, 156–169 (2012).
Guma, M., Ronacher, L. M., Firestein, G. S., Karin, M. & Corr, M. JNK-1 deficiency limits macrophage-mediated antigen-induced arthritis. Arthritis Rheum. 63, 1603–1612 (2011).
Denninger, K. et al. JNK1, but not JNK2, is required in two mechanistically distinct models of inflammatory arthritis. Am. J. Pathol. 179, 1884–1893 (2011).
Lee, S. I., Boyle, D. L., Berdeja, A. & Firestein, G. S. Regulation of inflammatory arthritis by the upstream kinase mitogen activated protein kinase kinase 7 in the c-Jun N-terminal kinase pathway. Arthritis Res. Ther. 14, R38 (2012).
Haruta, K. et al. Inhibitory effects of ZSTK474, a phosphatidylinositol 3-kinase inhibitor, on adjuvant-induced arthritis in rats. Inflamm. Res. 61, 551–562 (2012).
Cha, H. S. et al. A novel spleen tyrosine kinase inhibitor blocks c-Jun N-terminal kinase-mediated gene expression in synoviocytes. J. Pharmacol. Exp. Ther. 317, 571–578 (2006).
Weinblatt, M. E. et al. An oral spleen tyrosine kinase (Syk) inhibitor for rheumatoid arthritis. N. Engl. J. Med. 363, 1303–1312 (2010).
Schieven, G. L. The p38α kinase plays a central role in inflammation. Curr. Top. Med. Chem. 9, 1038–1048 (2009).
Damjanov, N., Kauffman, R. S. & Spencer-Green, G. T. Efficacy, pharmacodynamics, and safety of VX-702, a novel p38 MAPK inhibitor, in rheumatoid arthritis: results of two randomized, double-blind, placebo-controlled clinical studies. Arthritis Rheum. 60, 1232–1241 (2009).
Inoue, T. et al. Mitogen-activated protein kinase kinase 3 is a pivotal pathway regulating p38 activation in inflammatory arthritis. Proc. Natl Acad. Sci. USA 103, 5484–5489 (2006).
Hah, Y. S. et al. A20 suppresses inflammatory responses and bone destruction in human fibroblast-like synoviocytes and in mice with collagen-induced arthritis. Arthritis Rheum. 62, 2313–2321 (2010).
Tak, P. P. et al. Inhibitor of nuclear factor κB kinase β is a key regulator of synovial inflammation. Arthritis Rheum. 44, 1897–1907 (2001).
Shi, J. et al. Epirubicin potentiates recombinant adeno-associated virus type 2/5-mediated TRAIL expression in fibroblast-like synoviocytes and augments the antiarthritic effects of rAAV2/5-TRAIL. Arthritis Rheum. 64, 1345–1354 (2012).
Lin, H. S. et al. Anti-rheumatic activities of histone deacetylase (HDAC) inhibitors in vivo in collagen-induced arthritis in rodents. Br. J. Pharmacol. 150, 862–872 (2007).
Kavanaugh, A. F. et al. Treatment of refractory rheumatoid arthritis with a monoclonal antibody to intercellular adhesion molecule 1. Arthritis Rheum. 37, 992–999 (1994).
Acknowledgements
The authors are indebted to M. Bottini and S. Stanford for help with preparation of the figures. This work was supported, in part, by Institutional La Jolla Institute of Allergy and Immunology funds (to N. Bottini) and by NIH grants R01AI067752, R01 AI070555, and R01 AR47825 (to G. S. Firestein). This manuscript is #1556 published from the La Jolla Institute of Allergy and Immunology.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to researching data for the article, discussing its content, writing, and review/editing of the manuscript before publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Bottini, N., Firestein, G. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol 9, 24–33 (2013). https://doi.org/10.1038/nrrheum.2012.190
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2012.190