Key Points
-
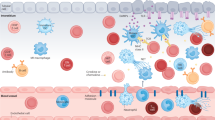
Sterile inflammation occurs in organs after their surgical removal and implantation into a recipient
-
Inflammation that occurs after solid organ transplantation can precipitate acute allograft rejection, impede transplant tolerance and enhance the development of chronic allograft rejection
-
Experimental and clinical studies have shown that several endogenous substances, also known as damage associated molecular patterns contribute to both acute and chronic allograft rejection
-
Toll-like receptors, which are among the best characterized innate immune receptors, induce inflammation and impair outcomes after solid organ transplantation
-
Clinical studies are investigating strategies to inhibit innate immune responses after organ transplantation; approaches to reduce inflammation without compromising host defence to pathogens would substantially improve outcomes for transplant recipients
Abstract
Graft inflammation impairs the induction of solid organ transplant tolerance and enhances acute and chronic rejection. Elucidating the mechanisms by which inflammation is induced after organ transplantation could lead to novel therapeutics to improve transplant outcomes. In this Review we describe endogenous substances — damage-associated molecular patterns (DAMPs) — that are released after allograft reperfusion and induce inflammation. We also describe innate immune signalling pathways that are activated after solid organ transplantation, with a focus on Toll-like receptors (TLRs) and their signal adaptor, MYD88. Experimental and clinical studies have yielded a large body of evidence that TLRs and MYD88 are instrumental in initiating allograft inflammation and promoting the development of acute and chronic rejection. Ongoing clinical studies are testing TLR inhibition strategies in solid organ transplantation, although avoiding compromising host defence to pathogens is a key challenge. Further elucidation of the mechanisms by which sterile inflammation is induced, maintained and amplified within the allograft has the potential to lead to novel anti-inflammatory treatments that could improve outcomes for solid organ transplant recipients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Wu, H. & Chadban, S. J. Roles of Toll-like receptors in transplantation. Curr. Opin. Organ Transplant. 19, 1–7 (2014).
Mori, D. N., Kreisel, D., Fullerton, J. N., Gilroy, D. W. & Goldstein, D. R. Inflammatory triggers of acute rejection of organ allografts. Immunol. Rev. 258, 132–144 (2014).
Chong, A. & Alegre, M. The impact of infection and tissue damage in solid-organ transplantation. Nat. Rev. Immunol. 12, 459–471 (2012).
McFarland-Mancini, M. M. et al. Differences in wound healing in mice with deficiency of IL-6 versus IL-6 receptor. J. Immunol. 184, 7219–7228 (2010).
Cameron, G. D. et al. Haptoglobin phenotype correlates with development of cardiac transplant vasculopathy. J. Heart Lung Transplant. 23, 43–49 (2004).
Shirali, A. & Goldstein, D. Tracking the toll of kidney disease. J. Am. Soc. Nephrol. 19, 1444–1450 (2008).
Shirali, A. & Goldstein, D. R. Activation of the innate immune system by the endogenous ligand hyaluronan. Curr. Opin. Organ Transplant. 13, 20–25 (2008).
Chen, G. Y. & Nuñez, G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 10, 826–837 (2010).
Gjertson, D. Impact of delayed graft function and acute rejection on kidney graft survival. Clin. Transpl. 467–480 (2000).
Debout, A. et al. Each additional hour of cold ischemia time significantly increases the risk of graft failure and mortality following renal transplantation. Kidney Int. 87, 343–349 (2015).
Stehlik, J. et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-eighth adult heart transplant report — 2011. J. Heart Lung Transplant. 30, 1078–1094 (2011).
Lund, L. H. et al. The Registry of the International Society for Heart and Lung Transplantation: thirty-second official adult heart transplantation report — focus theme: early graft failure. J. Heart Lung Transplant. 34, 1244–1254 (2015).
Chalasani, G. et al. The allograft defines the type of rejection (acute versus chronic) in the face of an established effector immune response. J. Immunol. 172, 7813–7820 (2004).
Ponticelli, C. Ischaemia-reperfusion injury: a major protagonist in kidney transplantation. Nephrol. Dial. Transplant. 29, 1134–1140 (2014).
Matas, A. J. et al. 2202 kidney transplant recipients with 10 years of graft function: what happens next? Am. J. Trans. 8, 2410–2419 (2008).
Floerchinger, B. et al. Inflammatory immune responses in a reproducible mouse brain death model. Transpl. Immunol. 27, 25–29 (2012).
Hoffmann, S. C. et al. Molecular and immunohistochemical characterization of the onset and resolution of human renal allograft ischemia-reperfusion injury. Transplantation 74, 916–923 (2002).
Sheen, J. H. & Heeger, P. S. Effects of complement activation on allograft injury. Curr. Opin. Organ Transplant. 20, 468–475 (2015).
Ali, F., Dua, A. & Cronin, D. C. Changing paradigms in organ preservation and resuscitation. Curr. Opin. Organ Transplant. 20, 152–158 (2015).
Li, W. et al. Intravital 2-photon imaging of leukocyte trafficking in beating heart. J. Clin. Invest. 122, 2499–2508 (2012).
Pittman, K. & Kubes, P. Damage-associated molecular patterns control neutrophil recruitment. J. Innate Immun. 5, 315–323 (2013).
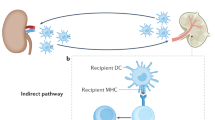
Zhuang, Q. & Lakkis, F. G. Dendritic cells and innate immunity in kidney transplantation. Kidney Int. 5, 315–3 (2015).
Lakkis, F. G. Where is the alloimmune response initiated? Am. J. Transplant 3, 241–242 (2003).
Tsung, A. et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J. Exp. Med. 201, 1135–1143 (2005).
Wu, H. et al. HMGB1 contributes to kidney ischemia reperfusion injury. J. Am. Soc. Nephrol. 21, 1878–1890 (2010).
Rabadi, M. M., Ghaly, T., Goligorksy, M. S. & Ratliff, B. B. HMGB1 in renal ischemic injury. Am. J. Physiol. Renal Physiol. 303, F873–F885 (2012).
Kamo, N. et al. ASC/caspase-1/IL-1β signaling triggers inflammatory responses by promoting HMGB1 induction in liver ischemia/reperfusion injury. Hepatology 58, 351–362 (2013).
Tsung, A. et al. HMGB1 release induced by liver ischemia involves Toll-like receptor 4-dependent reactive oxygen species production and calcium-mediated signaling. J. Exp. Med. 204, 2913–2923 (2007).
Helena Erlandsson Harris, U. A. Mini-review: the nuclear protein HMGB1 as a proinflammatory mediator. Eur. J. Immunol. 34, 1503–1512 (2004).
Zhang, A. et al. Necrostatin-1 inhibits Hmgb1-IL-23/IL-17 pathway and attenuates cardiac ischemia reperfusion injury. Transpl. Int. 27, 1077–1085 (2014).
Syrjälä, S. O. et al. Increased Th17 rather than Th1 alloimmune response is associated with cardiac allograft vasculopathy after hypothermic preservation in the rat. J. Heart Lung Transplant. 29, 1047–1057 (2010).
Huang, Y. et al. Extracellular hmgb1 functions as an innate immune-mediator implicated in murine cardiac allograft acute rejection. Am. J. Transplant. 7, 799–808 (2007).
Moser, B. et al. Blockade of RAGE suppresses alloimmune reactions in vitro and delays allograft rejection in murine heart transplantation. Am. J. Transplant. 7, 293–302 (2007).
Dessing, M. C. et al. RAGE does not contribute to renal injury and damage upon ischemia/reperfusion-induced injury. J. Innate Immun. 4, 80–85 (2012).
Li, J. H. et al. Blockade of extracellular HMGB1 suppresses xenoreactive B cell responses and delays acute vascular xenogeneic rejection. Am. J. Transplant. 15, 2062–2074 (2015).
Zou, H. et al. HMGB1 is involved in chronic rejection of cardiac allograft via promoting inflammatory-like mDCs. Am. J. Transplant. 14, 1765–1777 (2014).
Hausenloy, D. J. & Yellon, D. M. Ischaemic conditioning and reperfusion injury. Nat. Rev. Cardiol. http://dx.doi.org/10.1038/nrcardio.2016.5 (2016).
Wu, H. et al. Preconditioning with recombinant high-mobility group box 1 protein protects the kidney against ischemia-reperfusion injury in mice. Kidney Int. 85, 824–832 (2014).
Netea, Mihai, G., Quintin, J. & van der Meer, Jos, W. M. Trained immunity: a memory for innate host defense. Cell Host Microbe 9, 355–361 (2011).
Jiang, D. et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat. Med. 11, 1173–1179 (2005).
Teder, P. et al. Resolution of lung inflammation by CD44. Science 296, 155–158 (2002).
Noble, P. W., McKee, C. M., Cowman, M. & Shin, H. S. Hyaluronan fragments activate an NF-κB/I-κBα autoregulatory loop in murine macrophages. J. Exp. Med. 183, 2373–2378 (1996).
Rouschop, K. M. A. et al. Protection against renal ischemia reperfusion injury by CD44 disruption. J. Am. Soc. Nephrol. 16, 2034–2043 (2005).
Wu, H. et al. TLR4 activation mediates kidney ischemia/reperfusion injury. J. Clin. Invest. 117, 2847–2859 (2007).
Scheibner, K. A. et al. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J. Immunol. 177, 1272–1281 (2006).
Bollyky, P. L. et al. Cutting edge: high molecular weight hyaluronan promotes the suppressive effects of CD4+CD25+ regulatory T cells. J. Immmunol. 179, 744–747 (2007).
Muto, J. et al. Hyaluronan digestion controls DC migration from the skin. J. Clin. Invest. 124, 1309–1319 (2014).
Wells, A. et al. Increased hyaluronan in acutely rejecting human kidney grafts. Transplantation 55, 1346–1349 (1993).
Tesar, B. M. et al. The role of hyaluronan degradation products as innate alloimmune agonists. Am. J. Transplant. 6, 2622–2635 (2006).
Todd, J. L. et al. Hyaluronan contributes to bronchiolitis obliterans syndrome and stimulates lung allograft rejection through activation of innate immunity. Am. J. Respir. Crit. Care Med. 189, 556–566 (2014).
Kono, H., Chen, C.-J., Ontiveros, F. & Rock, K. L. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J. Clin. Invest. 120, 1939–1949 (2010).
Shen, H., Kreisel, D. & Goldstein, D. R. Processes of sterile inflammation. J. Immunol. 191, 2857–2863 (2013).
Oh, K.-H. et al. Targeted gene disruption of the heat shock protein 72 gene (hsp70.1) in the donor tissue is associated with a prolonged rejection-free survival in the murine skin allograft model. Transplant Immunol. 13, 273–281 (2004).
Tesar, B. & Goldstein, D. R. Acute allograft rejections occurs independently of inducible HSP-70. Transplantation 11, 1513–1517 (2007).
Quaye, I. K. Haptoglobin, inflammation and disease. Trans. R. Soc. Trop. Med. Hyg. 102, 735–742 (2008).
Shen, H. et al. Haptoglobin activates innate immunity to enhance acute transplant rejection in mice. J. Clin. Invest. 122, 383–387 (2012).
Goldstein, D. R., Tesar, B. M., Akira, S. & Lakkis, F. G. Critical role of the Toll-like receptor signal adaptor protein MyD88 in acute allograft rejection. J. Clin. Invest. 111, 1571–1578 (2003).
Shen, H. et al. Haptoglobin enhances cardiac transplant rejection. Circ. Res. 116, 1670–1679 (2015).
Brubaker, S. W., Bonham, K. S., Zanoni, I. & Kagan, J. C. Innate immune pattern recognition: a cell biological perspective. Annu. Rev. Immunol. 33, 257–290 (2015).
Guo, H., Callaway, J. B. & Ting, J. P. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat. Med. 21, 677–687 (2015).
Yoneyama, M., Onomoto, K., Jogi, M., Akaboshi, T. & Fujita, T. Viral RNA detection by RIG-I-like receptors. Curr. Opin. Immunol. 32, 48–53 (2015).
Fitzgerald, M. E., Rawling, D. C., Vela, A. & Pyle, A. M. An evolving arsenal: viral RNA detection by RIG-I-like receptors. Curr. Opin. Microbiol. 20, 76–81 (2014).
Seto, T. et al. Upregulation of the apoptosis-related inflammasome in cardiac allograft rejection. J. Heart Lung Transplant. 29, 352–359 (2010).
Shah, K. B., Mauro, A. G., Flattery, M., Toldo, S. & Abbate, A. Formation of the inflammasome during cardiac allograft rejection. Int. J. Cardiol. 201, 328–330 (2015).
Pandey, S., Kawai, T. & Akira, S. Microbial sensing by Toll-like receptors and intracellular nucleic acid sensors. Cold Spring Harb. Perspect. Biol. 7, a016246 (2015).
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010).
Crellin, N. K. et al. Regulation of cytokine secretion in human CD127+ LTi-like innate lymphoid cells by Toll-like receptor 2. Immunity 33, 752–764 (2010).
Leemans, J. C. et al. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J. Clin. Invest. 115, 2894–2903 (2005).
Patole, P. S. et al. Toll-like receptor-4: renal cells and bone marrow cells signal for neutrophil recruitment during pyelonephritis. Kidney Int. 68, 2582–2587 (2005).
Leventhal, J. S. & Schroppel, B. Toll-like receptors in transplantation: sensing and reacting to injury. Kidney Int. 81, 826–832 (2012).
Tesar, B. M., Zhang, J., Li, Q. & Goldstein, D. R. TH1 immune responses to fully MHC mismatched allografts are diminished in the absence of MyD88, a toll like receptor signal adaptor protein. Am. J. Transplant. 4, 1429–1439 (2004).
Walker, W. E. et al. Absence of innate MyD88 signaling promotes inducible allograft acceptance. J. Immunol. 177, 5307–5316 (2006).
Wu, H. et al. Absence of MyD88 signaling induces donor-specific kidney allograft tolerance. J. Am. Soc. Nephrol. 23, 1701–1716 (2012).
Kruger, B. et al. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc. Natl Acad. Sci. USA 106, 3390–3395 (2009).
Pulskens, W. P. et al. Toll-like receptor-4 coordinates the innate immune response of the kidney to renal ischemia/reperfusion injury. PLoS ONE 3, e3596 (2008).
Oberbarnscheidt, M. H. et al. Non-self recognition by monocytes initiates allograft rejection. J. Clin. Invest. 124, 3579–3589 (2014).
Chen, L. et al. TLR engagement prevents transplantation tolerance. Am. J. Trans. 6, 2282–2291 (2006).
Zhang, X. et al. Induction of alloimmune tolerance in heart transplantation through gene silencing of TLR adaptors. Am. J. Trans. 12, 2675–2688 (2012).
Kaczorowski, D. J. et al. Mechanisms of Toll-like receptor 4 (TLR4)-mediated inflammation after cold ischemia/reperfusion in the heart. Transplantation 87, 1455–1463 (2009).
Wang, S. et al. Recipient Toll-like receptors contribute to chronic graft dysfunction by both MyD88- and TRIF-dependent signaling. Dis. Model. Mech. 3, 92–103 (2010).
Thierry, A. et al. The alarmin concept applied to human renal transplantation: evidence for a differential implication of HMGB1 and IL-33. PLoS ONE 9, e88742 (2014).
Ilmakunnas, M. et al. High mobility group box 1 protein as a marker of hepatocellular injury in human liver transplantation. Liver Transplant. 14, 1517–1525 (2008).
Cantu, E. et al. Gene set enrichment analysis identifies key innate immune pathways in primary graft dysfunction after lung transplantation. Am. J. Trans. 13, 1898–1904 (2013).
Andrade, C. F. et al. Toll-like receptor and cytokine gene expression in the early phase of human lung transplantation. J. Heart Lung Transplant. 25, 1317–1323 (2006).
Palmer, S. M. et al. Donor polymorphisms in Toll-like receptor-4 influence the development of rejection after renal transplantation. Clin. Transplant. 20, 30–36 (2006).
Dhillon, N. et al. A single nucleotide polymorphism of Toll-like receptor 4 identifies the risk of developing graft failure after liver transplantation. J. Hepatol. 53, 67–72 (2010).
Dessing, M. C. et al. Intragraft Toll-like receptor profiling in acute renal allograft rejection. Nephrol. Dial. Transplant. 25, 4087–4092 (2010).
Dessing, M. C. et al. Toll-like receptor family polymorphisms are associated with primary renal diseases but not with renal outcomes following kidney transplantation. PLoS ONE 10, e0139769 (2015).
Kuhlicke, J., Frick, J. S., Morote-Garcia, J. C., Rosenberger, P. & Eltzschig, H. K. Hypoxia inducible factor (HIF)-1 coordinates induction of Toll-like receptors TLR2 and TLR6 during hypoxia. PLoS ONE 2, e1364 (2007).
Powers, K. A. et al. Oxidative stress generated by hemorrhagic shock recruits Toll-like receptor 4 to the plasma membrane in macrophages. J. Exp. Med. 203, 1951–1961 (2006).
Zarember, K. A. & Godowski, P. J. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J. Immunol. 168, 554–561 (2002).
Calfee, C. S. et al. Plasma receptor for advanced glycation end-products predicts duration of ICU stay and mechanical ventilation in patients after lung transplantation. J. Heart Lung Transplant. 26, 675–680 (2007).
Oetting, W. S. et al. Donor polymorphisms of toll-like receptor 4 associated with graft failure in liver transplant recipients. Liver Transpl. 18, 1399–1405 (2012).
Kastelijn, E. A. et al. Polymorphisms in innate immunity genes associated with development of bronchiolitis obliterans after lung transplantation. J. Heart Lung Transplant. 29, 665–671 (2010).
Ducloux, D. et al. Relevance of Toll-like receptor-4 polymorphisms in renal transplantation. Kidney Int. 67, 2454–2461 (2005).
Palmer, C., Diehn, M., Alizadeh, A. A. & Brown, P. O. Cell-type specific gene expression profiles of leukocytes in human peripheral blood. BMC Genomics 7, 115–115 (2006).
Park, W. D., Griffin, M. D., Cornell, L. D., Cosio, F. G. & Stegall, M. D. Fibrosis with inflammation at one year predicts transplant functional decline. J. Am. Soc. Nephrol. 21, 1987–1997 (2010).
Park, W., Griffin, M., Grande, J. P., Cosio, F. & Stegall, M. D. Molecular evidence of injury and inflammation in normal and fibrotic renal allografts one year posttransplant. Transplantation 83, 1466–1476 (2007).
Braudeau, C. et al. Contrasted blood and intragraft toll-like receptor 4 mRNA profiles in operational tolerance versus chronic rejection in kidney transplant recipients. Transplantation 86, 130–136 (2008).
Methe, H., Zimmer, E., Grimm, C., Nabauer, M. & Koglin, J. Evidence for a role of toll-like receptor 4 in development of chronic allograft rejection after cardiac transplantation. Transplantation 78, 1324–1331 (2004).
Naesens, M. et al. Progressive histological damage in renal allografts is associated with expression of innate and adaptive immunity genes. Kidney Int. 80, 1364–1376 (2011).
Khatri, P. et al. A common rejection module (CRM) for acute rejection across multiple organs identifies novel therapeutics for organ transplantation. J. Exp. Med. 210, 2205–2221 (2013).
Saito, T. et al. Distinct expression patterns of alveolar “alarmins” in subtypes of chronic lung allograft dysfunction. Am. J. Trans. 14, 1425–1432 (2014).
Chan, J. K. et al. Alarmins: awaiting a clinical response. J. Clin. Invest. 122, 2711–2719 (2012).
Eikmans, M. et al. Expression of surfactant protein-C, S100A8, S100A9, and B cell markers in renal allografts: investigation of the prognostic value. J. Soc. Am. Neprol 16, 3771–3786 (2005).
Dessing, M. C. et al. The calcium-binding protein complex S100A8/A9 has a crucial role in controlling macrophage-mediated renal repair following ischemia/reperfusion. Kidney Int. 87, 85–94 (2015).
Shimizu, K. et al. Loss of myeloid related protein-8/14 exacerbates cardiac allograft rejection. Circulation 124, 2920–2932 (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
Soares, M. P., Gozzelino, R. & Weis, S. Tissue damage control in disease tolerance. Trends Immunol. 35, 483–494 (2014).
Anders, H.-J. Immune system modulation of kidney regeneration[mdash]mechanisms and implications. Nat. Rev. Nephrol. 10, 347–358 (2014).
Serhan, C. N., Chiang, N. & Van Dyke, T. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 8, 349–361 (2008).
Kim, M.-G. et al. The heat-shock protein-70-induced renoprotective effect is partially mediated by CD4+CD25+Foxp3+ regulatory T cells in ischemia/reperfusion-induced acute kidney injury. Kidney Int. 85, 62–71 (2014).
Weidemann, A. & Johnson, R. S. Biology of HIF-1α. Cell Death Differ. 15, 621–627 (2008).
Kido, M. et al. Hypoxia-inducible factor 1-alpha reduces infarction and attenuates progression of cardiac dysfunction after myocardial infarction in the mouse. J. Am. College Cardiol. 46, 2116–2124 (2005).
Cai, Z. et al. Complete loss of ischaemic preconditioning-induced cardioprotection in mice with partial deficiency of HIF-1α. Cardiovasc. Res. 77, 463–470 (2008).
Eltzschig, H. K. & Eckle, T. Ischemia and reperfusion — from mechanism to translation. Nat. Med. 17, 1391–1401 (2011).
Medzhitov, R., Schneider, D. S. & Soares, M. P. Disease tolerance as a defense strategy. Science 335, 936–941 (2012).
Chen, Y. et al. Remote ischemic preconditioning fails to improve early renal function of patients undergoing living-donor renal transplantation: a randomized controlled trial. Transplantation 95, e4–6 (2013).
Hill, P. et al. Inhibition of hypoxia inducible factor hydroxylases protects against renal ischemia-reperfusion injury. J. Am. Soc. Nephrol. 19, 39–46 (2008).
Ferenbach, D. A. et al. The induction of macrophage hemeoxygenase-1 is protective during acute kidney injury in aging mice. Kidney Int. 79, 966–976 (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
Medzhitov, R. & Janeway, C. Jr. Innate immunity. N. Engl. J. Med. 343, 338–344 (2000).
Acknowledgements
D.R.G.'s work is supported by NIH/NIA grant R01AG028082, S.J.C.'s work is supported by NHMRC Project Grant funding and S.B.'s work is supported by the CENTAURE foundation, the IHU-Cesti project, the French National Research Agency, and the Nantes Metropole and the Pays de la Loire Region.
Author information
Authors and Affiliations
Contributions
All authors researched the data, discussed the content, wrote the article, and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
PowerPoint slides
Glossary
- Sterile inflammation
-
Inflammation that occurs following the necrosis- mediated release of activators of inflammation in medical conditions such as ischaemia–reperfusion injury, crystalline-induced arthritis, acute lung injury and chronic inflammatory conditions, without an identifiable infectious precipitant.
- Bronchiolitis obliterans syndrome
-
A manifestation of chronic allograft rejection characterized by a decline in pulmonary function that affects more than half of lung recipients 5 years after transplantation and accounts for a considerable proportion of lung allograft losses and recipient deaths.
- Operationally tolerant recipients
-
Subgroup of patients who spontaneously tolerate their graft and maintain allograft function without the use of immunosuppressants for at least 1 year, in the absence of deleterious responses.
- Restrictive allograft syndrome
-
Newly described phenotype of chronic lung allograft dysfunction characterized by a persistentdecline in vital and total lung capacities and allograft parenchymal fibrosis.
Rights and permissions
About this article
Cite this article
Braza, F., Brouard, S., Chadban, S. et al. Role of TLRs and DAMPs in allograft inflammation and transplant outcomes. Nat Rev Nephrol 12, 281–290 (2016). https://doi.org/10.1038/nrneph.2016.41
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2016.41