Key Points
-
There is abundant experimental evidence that the sex hormone oestrogen might have neuroprotective actions. Observations in vivo and in vitro, as well as epidemiological studies, lend general support to this idea.
-
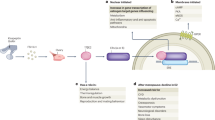
There are several possible mechanisms to account for the neuroprotective actions of oestrogen. In the simplest scenario, oestrogen receptors, which are transcription factors, might act directly on genes that code for proteins that modulate nerve-cell survival, regulating their expression. These proteins might enhance neurotrophic support, suppress apoptosis and affect neuronal structure.
-
The neuroprotective action of oestrogen could also depend on non-classical actions of this neurohormone. Indeed, oestrogen can interact with intracellular signalling pathways that are directly linked to the control of neuronal survival, such as the mitogen-activated protein kinase (MAPK) pathway, cyclic-AMP-responsive-element-binding protein (CREB) and phosphatidylinositol 3-kinase (PI3K). In addition, the chemical structure of oestrogen enables it to act as a free-radical scavenger, preventing oxidative damage.
Abstract
In addition to its role as a sex hormone, oestrogen affects the structure and function of the nervous system. Oestrogen receptors are expressed in brain regions that are involved in sex differentiation and maturation. But in addition to its well-known effects, oestrogen also has important neuroprotective actions that are both dependent and independent of a nuclear oestrogen-receptor activity. Furthermore, oestrogen can interact with neuroprotective intracellular signalling pathways and is itself a neuroprotective antioxidant. Understanding the mechanisms of oestrogen action will be crucial to determine its potential as a therapeutic agent, particularly in the elderly.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Payami, H. et al. Gender difference in apolipoprotein E-associated risk for familial Alzheimer disease – a possible clue to the higher incidence of Alzheimer disease in women. Am. J. Hum. Genet. 58, 803–811 (1996).
Roof, R. L. & Hall, E. D. Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J. Neurotrauma 17, 367–388 (2000).
Takahashi, S. et al. Age at onset of schizophrenia: gender differences and influence of temporal socioeconomic change. Psychiatry Clin. Neurosci. 54, 153–156 (2000).
Nolen-Hoeksema, S. Gender differences in depression. Curr. Dir. Psychol. Sci. 10, 173–176 (2001).
McEwen, B. S. Estrogen effects on the brain: multiple sites and molecular mechanisms. J. Appl. Physiol. 91, 2785–2801 (2001).A recent authoritative review on the multiple roles of oestrogen in the brain.
Bishop, J. & Simpkins, J. W. Estradiol treatment increases viability of glioma and neuroblastoma cells in vitro. Mol. Cell. Neurosci. 5, 303–308 (1994).
Behl, C., Widmann, M., Trapp, T. & Holsboer, F. 17-β Estradiol protects neurons from oxidative stress-induced cell death in vitro. Biochem. Biophys. Res. Commun. 216, 473–482 (1995).
Green, P. S. & Simpkins, J. W. Neuroprotective effects of estrogens: potential mechanisms of action. Int. J. Dev. Neurosci. 18, 347–358 (2000).
Wise, P. M. et al. Estradiol is a protective factor in the adult and aging brain: understanding of mechanisms derived from in vivo and in vitro studies. Brain Res. Brain Res. Rev. 37, 313–319 (2001).
Finkel, T. & Holbrook, N. J. Oxidants, oxidative stress and the biology of aging. Nature 408, 239–247 (2000).
Behl, C. & Moosmann, B. Oxidative nerve cell death in Alzheimer's disease and stroke: antioxidants as neuroprotective compounds. Biol. Chem. 383, 521–536 (2002).
Hurn, P. D. & Macrae, I. M. Estrogen as a neuroprotectant in stroke. J. Cereb. Blood Flow Metab. 20, 631–652 (2000).
Freyaldenhoven, T. E., Cadet, J. L. & Ali, S. F. The dopamine-depleting effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in cd-1 mice are gender-dependent. Brain Res. 735, 232–238 (1996).
Hossmann, K. A. Experimental models for the investigation of brain ischemia. Cardiovasc. Res. 39, 106–120 (1998).
Alkayed, N. J. et al. Gender-linked brain injury in experimental stroke. Stroke 29, 159–165 (1998).
Zhang, Y. Q., Shi, J., Rajakumar, G., Day, A. L. & Simpkins, J. W. Effects of gender and estradiol treatment on focal brain ischemia. Brain Res. 784, 321–324 (1998).
Saleh, T. M., Cribb, A. E. & Connell, B. J. Estrogen-induced recovery of autonomic function after middle cerebral artery occlusion in male rats. Am J Physiol Regul Integr Comp Physiol 281, R1531–R1539 (2001).
Barrett-Connor, E. & Bush, T. L. Estrogen and coronary heart disease in women. JAMA 265, 1861–1867 (1991).
Shi, J. et al. Estrogens decrease reperfusion-associated cortical ischemic damage – an MRI analysis in a transient focal ischemia model. Stroke 32, 987–992 (2001).
Jover, T. et al. Estrogen protects against global ischemia-induced neuronal death and prevents activation of apoptotic signaling cascades in the hippocampal CA1. J. Neurosci. 22, 2115–2124 (2002).Shows that long-term treatment with oestrogen at physiological levels ameliorates ischaemia-induced hippocampal injury.
Yang, S. H., Shi, J., Day, A. L. & Simpkins, J. W. Estradiol exerts neuroprotective effects when administered after ischemic insult. Stroke 31, 745–749 (2000).Oestrogen is neuroprotective even when applied after the onset of ischaemic damage.
Dluzen, D. E. & McDermott, J. L. Gender differences in neurotoxicity of the nigrostriatal dopaminergic system: implications for Parkinson's disease. J. Gend. Specif. Med. 3, 36–42 (2000).
Beal, M. F. Experimental models of Parkinson's disease. Nature Rev. Neurosci. 2, 325–332 (2001).
Kuppers, E., Ivanova, T., Karolczak, M. & Beyer, C. Estrogen: a multifunctional messenger to nigrostriatal dopaminergic neurons. J. Neurocytol. 29, 375–385 (2000).
Grandbois, M., Morissette, M., Callier, S. & Di Paolo, T. Ovarian steroids and raloxifene prevent MPTP-induced dopamine depletion in mice. Neuroreport 11, 343–346 (2000).
Azcoitia, I., Sierra, A. & Garcia-Segura, L. M. Estradiol prevents kainic acid-induced neuronal loss in the rat dentate gyrus. Neuroreport 9, 3075–3079 (1998).
Yankova, M., Hart, S. A. & Woolley, C. S. Estrogen increases synaptic connectivity between single presynaptic inputs and multiple postsynaptic CA1 pyramidal cells: a serial electron-microscopic study. Proc. Natl Acad. Sci. USA 98, 3525–3530 (2001).
Foy, M. R. 17β-Estradiol: effect on CA1 hippocampal synaptic plasticity. Neurobiol. Learn. Mem. 76, 239–252 (2001).
Gould, E. & Gross, C. G. Neurogenesis in adult mammals: some progress and problems. J. Neurosci. 22, 619–623 (2002).
Tanapat, P., Hastings, N. B., Reeves, A. J. & Gould, E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J. Neurosci. 19, 5792–5801 (1999).
Cameron, H. A. & McKay, R. D. G. Restoring production of hippocampal neurons in old age. Nature Neurosci. 2, 894–897 (1999).
Brinton, R. D. et al. The Women's Health Initiative estrogen replacement therapy is neurotrophic and neuroprotective. Neurobiol. Aging 21, 475–496 (2000).
Seeman, M. V. Psychopathology in women and men – focus on female hormones. Am. J. Psychiatry 154, 1641–1647 (1997).
Henderson, V. W. Oestrogens and dementia. Novartis Found. Symp. 230, 254–273 (2000).
Paganini-Hill, A. Estrogen replacement therapy and stroke. Prog. Cardiovasc. Dis. 38, 223–242 (1995).
Gail, M. H. et al. Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer – response. J. Natl Cancer Inst. 91, 1829–1846 (1999).
Paganini-Hill, A. Hormone replacement therapy and stroke: risk, protection or no effect? Maturitas 38, 243–261 (2001).
Sherwin, B. B. Can estrogen keep you smart? Evidence from clinical studies. J. Psychiatry Neurosci. 24, 315–321 (1999).This comprehensive review discusses the biological plausibility and the clinical empirical evidence of a link between oestrogen levels and memory in women.
Paganini-Hill, A. & Henderson, V. W. Estrogen replacement therapy and risk of Alzheimer Disease. Arch. Intern. Med. 156, 2213–2217 (1996).
Kawas, C. et al. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer's disease: the Baltimore Longitudinal Study of Aging. Neurology 48, 1517–1521 (1997).
Gao, S., Hendrie, H. C. & Hall, K. S. The relationship between age, sex, and the incidence of dementia and Alzheimer Disease – a meta-analysis. Arch. Gen. Psychiatry 55, 809–815 (1998).This meta-analysis concludes that women are at higher risk of Alzheimer's disease than men.
Carlson, M. C. et al. Hormone replacement therapy and reduced cognitive decline in older women: the Cache County Study. Neurology 57, 2210–2216 (2001).
Neurgaren, B. L. & Kraines, R. J. Menopausal symptoms in women of various ages. Psychol. Med. 27, 266–273 (1965).
Tang, M. X. et al. Effect of oestrogen during menopause on risk and age of onset of Alzheimer's Disease. Lancet 348, 429–432 (1996).
Slooter, A. J. C. et al. Estrogen use and early onset Alzheimer's disease: a population-based study. J. Neurol. Neurosurg. Psychiatry 67, 779–781 (1999).
Waring, S. C. et al. Postmenopausal estrogen replacement therapy and risk of AD – a population-based study. Neurology 52, 965–970 (1999).References 39, 42 and 44–46 argue in favour of ERT for the prevention of Alzheimer's disease.
Wang, P. N. et al. Effects of estrogen on cognition, mood, and cerebral blood flow in AD – a controlled study. Neurology 54, 2061–2066 (2000).
Mulnard, R. I. et al. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease – a randomized controlled trial. JAMA 283, 1007–1015 (2000).
Seshadri, S. et al. Postmenopausal estrogen replacement therapy and the risk of Alzheimer disease. Arch. Neurol. 58, 435–440 (2001).
Fillit, H. et al. Observations in a preliminary open trial of estradiol therapy for senile dementia–Alzheimer's type. Psychoneuroendocrinology 11, 337–345 (1986).Seven women with Alzheimer's disease were treated with low doses of oestrogen over six weeks. Significant improvements were noted in three women on measures of attention, orientation, mood and social interaction.
Henderson, V. W. et al. Estrogen for Alzheimer's disease in women – randomized, double-blind, placebo-controlled trial. Neurology 54, 295–301 (2000).References 47–49 and 51 argue against oestrogen replacement for Alzheimer's disease therapy.
Aranda, A. & Pascual, A. Nuclear hormone receptors and gene expression. Physiol. Rev. 81, 1269–1304 (2001).
Hall, J. M., Couse, J. F. & Korach, K. S. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J. Biol. Chem. 276, 36869–36872 (2001).
Jensen, E. V. & Jacobson, H. I. Basic guides to the mechanism of estrogen action. Recent Prog. Horm. Res. 18, 387–414 (1962).
Green, S. et al. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature 320, 134–139 (1986).
Greene, G. L. et al. Sequence and expression of human estrogen receptor complementary DNA. Science 231, 1150–1154 (1986).References 55 and 56 describe the cloning of the first human ER.
Kuiper, G. G., Enmark, E., Pelto-Huikko, M., Nilsson, S. & Gustafsson, J. A. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl Acad. Sci. USA 93, 5925–5930 (1996).This paper describes the cloning of ERβ. The discovery of ERβ is an important landmark in our understanding of oestrogen action in various tissues.
Couse, J. F. & Korach, K. S. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr. Rev. 20, 358–417 (1999).
Kuiper, G. G. J. M., Shughrue, P. J., Merchenthaler, I. & Gustafsson, J. A. The estrogen receptor β subtype – a novel mediator of estrogen action in neuroendocrine systems. Front. Neuroendocrinol. 19, 253–286 (1998).
Enmark, E. et al. Human estrogen receptor – gene structure, chromosomal localization, and expression pattern. J. Clin. Endocrinol. Metabol. 82, 4258–4265 (1997).
Shughrue, P. J., Lane, M. V. & Merchenthaler, I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J. Comp. Neurol. 388, 507–525 (1997).This comparative study provides evidence that the region-specific expression of ERα or ERβ, or both, could be important in determining the physiological responses of neuronal populations to oestrogen.
Shughrue, P. J., Scrimo, P. J. & Merchenthaler, I. Estrogen binding and estrogen receptor characterization (ERα and ERβ) in the cholinergic neurons of the rat basal forebrain. Neuroscience 96, 41–49 (2000).
Gudino-Cabrera, G. & Nieto-Sampedro, M. Estrogen receptor immunoreactivity in Schwann-like brain macroglia. J. Neurobiol. 40, 458–470 (1999).
Azcoitia, I., Garcia-Ovejero, D., Chowen, J. A. & Garcia-Segura, L. M. Astroglia play a key role in the neuroprotective actions of estrogen. Prog. Brain Res. 132, 469–478 (2001).
Santagati, S., Melcangi, R. C., Celotti, F., Martini, L. & Maggi, A. Estrogen receptor is expressed in different types of glial cells in culture. J. Neurochem. 63, 2058–2064 (1994).
Freedman, L. P. Multimeric coactivator complexes for steroid/nuclear receptors. Trends Endocrinol. Metab. 10, 403–407 (1999).
Klinge, C. M. Estrogen receptor interaction with co-activators and co-repressors. Steroids 65, 227–251 (2000).
Nilsson, S. et al. Mechanisms of estrogen action. Physiol. Rev. 81, 1535–1565 (2001).A recent review on the molecular mechanisms of oestrogen activity.
McKenna, N. J. & O'Malley, B. W. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108, 465–474 (2002).A detailed review on the mechanisms of signal transduction of nuclear receptors from pioneers in the field.
Pettersson, K., Grandien, K., Kuiper, G. G. J. M. & Gustafsson, J. A. Mouse estrogen receptor β forms estrogen response element-binding heterodimers with estrogen receptor α. Mol. Endocrinol. 11, 1486–1496 (1997).
Donaghue, C., Westley, B. R. & May, F. E. B. Selective promoter usage of the human estrogen receptor-α gene and its regulation by estrogen. Mol. Endocrinol. 13, 1934–1950 (1999).
Sohrabji, F., Miranda, R. C. G. & Toran-Allerand, C. D. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc. Natl Acad. Sci. USA 92, 11110–11114 (1995).
Toran-Allerand, C. D., Singh, M. & Setalo, G. Novel mechanisms of estrogen action in the brain: new players in an old story. Front. Neuroendocrinol. 20, 97–121 (1999).
Blurton-Jones, M. M., Roberts, J. A. & Tuszynski, M. H. Estrogen receptor immunoreactivity in the adult primate brain: neuronal distribution and association with p75, trkA, and choline acetyltransferase. J. Comp. Neurol. 405, 529–542 (1999).
Mattson, M. P. Apoptosis in neurodegenerative disorders. Nature Rev. Mol. Cell Biol. 1, 120–129 (2000).
Antonsson, B. Bax and other pro-apoptotic Bcl-2 family 'killer-proteins' and their victim, the mitochondrion. Cell Tissue Res. 306, 347–361 (2001).
Pike, C. J. Estrogen modulates neuronal Bcl-xL expression and β-amyloid-induced apoptosis: relevance to Alzheimer's disease. J. Neurochem. 72, 1552–1563 (1999).
Singer, C. A., Rogers, K. L. & Dorsa, D. M. Modulation of bcl-2 expression – a potential component of estrogen protection in NT2 neurons. Neuroreport 9, 2565–2568 (1998).
Alkayed, N. J. et al. Estrogen and Bcl-2: gene induction and effect of transgene in experimental stroke. J. Neurosci. 21, 7543–7550 (2001).
Zhang, Y., Tounekti, O., Akerman, B., Goodyer, C. G. & LeBlanc, A. A. 17-β-Estradiol induces an inhibitor of active caspases. J. Neurosci. 21, RC176 (2001).
Belcredito, S. et al. Estrogen neuroprotection: the involvement of the Bcl-2 binding protein BNIP2. Brain Res. Brain Res. Rev. 37, 335–342 (2001).
Ferreira, A. & Caceres, A. Estrogen-enhanced neurite growth: evidence for a selective induction of Tau and stable microtubules. J. Neurosci. 11, 392–400 (1991).
Scoville, S. A., Bufton, S. M. & Liuzzi, F. J. Estrogen regulates neurofilament gene expression in adult female rat dorsal root ganglion neurons. Exp. Neurol. 146, 596–599 (1997).
Brueggemeier, R. W. et al. 2-Methoxymethylestradiol: a new 2-methoxy estrogen analog that exhibits antiproliferative activity and alters tubulin dynamics. J. Steroid Biochem. Mol. Biol. 78, 145–156 (2001).
Shughrue, P. J. & Dorsa, D. M. Estrogen modulates the growth-associated protein GAP-43 (neuromodulin) mRNA in the rat preoptic area and basal hypothalamus. Neuroendocrinology 57, 439–447 (1993).
Lonard, D. M. & Smith, C. L. Molecular perspectives on selective estrogen receptor modulators (SERMs): progress in understanding their tissue-specific agonist and antagonist actions. Steroids 67, 15–24 (2002).
Singer, C. A., Figueroa-Masot, X. A., Batchelor, R. H. & Dorsa, D. M. The mitogen-activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. J. Neurosci. 19, 2455–2463 (1999).
Bi, R. F., Foy, M. R., Vouimba, R. M., Thompson, R. F. & Baudry, M. Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc. Natl Acad. Sci. USA 98, 13391–13395 (2001).
Manthey, D., Heck, S., Engert, S. & Behl, C. Estrogen induces a rapid secretion of amyloid β precursor protein via the mitogen-activated protein kinase pathway. Eur. J. Biochem. 268, 4285–4291 (2001).
Belcher, S. M. & Zsarnovszky, A. Estrogenic actions in the brain: estrogen, phytoestrogens, and rapid intracellular signaling mechanisms. J. Pharmacol. Exp. Ther. 299, 408–414 (2001).
Boonyaratanakornkit, V. et al. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol. Cell 8, 269–280 (2001).
Nethrapalli, I. S. et al. Estradiol (E2) elicits SRC phosphorylation in the mouse neocortex: the initial event in E2 activation of the MAPK cascade? Endocrinology 142, 5145–5148 (2001).
Setalo, G., Singh, M., Guan, X. P. & Toran-Allerand, C. D. Estradiol-induced phosphorylation of ERK1/2 in explants of the mouse cerebral cortex: the roles of heat shock protein 90 (Hsp90) and MEK2. J. Neurobiol. 50, 1–12 (2002).
Simoncini, T. et al. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 407, 538–541 (2000).
Xin, H. B. et al. Oestrogen protects FKBP12.6 null mice from cardiac hypertrophy. Nature 416, 334–337 (2002).
Kelly, M. J. & Wagner, E. J. Estrogen modulation of G-protein-coupled receptors. Trends Endocrinol. Metab. 10, 369–374 (1999).
Rupprecht, R. et al. Neuroactive steroids: molecular mechanisms of action and implications for neuropsychopharmacology. Brain Res. Brain Res. Rev. 37, 59–67 (2001).
Power, R. F., Mani, S. K., Codina, J., Conneely, O. M. & O'Malley, B. W. Dopaminergic and ligand-independent activation of steroid hormone receptors. Science 254, 1636–1639 (1991).
Coleman, K. M. & Smith, C. L. Intracellular signaling pathways: nongenomic actions of estrogens and ligand-independent activation of estrogen receptors. Front. Biosci. 6, D1379–D1391 (2001).
Pietras, R. J. & Szego, C. M. Cell membrane estrogen receptors resurface. Nature Med. 5, 1330 (1999).
Razandi, M., Pedram, A., Greene, G. L. & Levin, E. R. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol. Endocrinol. 13, 307–319 (1999).
Powell, C. E., Soto, A. M. & Sonnenschein, C. Identification and characterization of membrane estrogen receptor from MCF7 estrogen-target cells. J. Steroid Biochem. Mol. Biol. 77, 97–108 (2001).
Nadal, A. et al. Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor α and estrogen receptor β. Proc. Natl Acad. Sci. USA 97, 11603–11608 (2000).
Vasudevan, N., Kow, L. M. & Pfaff, D. W. Early membrane estrogenic effects required for full expression of slower genomic actions in a nerve cell line. Proc. Natl Acad. Sci. USA 98, 12267–12271 (2001).
Wetzel, C. H. R. et al. Functional antagonism of gonadal steroids at the 5-hydroxytryptamine type 3 receptor. Mol. Endocrinol. 12, 1441–1451 (1998).
Valverde, M. A. et al. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the β subunit. Science 285, 1929–1931 (1999).
Woolley, C. S. Electrophysiological and cellular effects of estrogen on neuronal function. Crit. Rev. Neurobiol. 13, 1–20 (1999).A comprehensive review on oestrogen's potential to modulate neurotransmission.
Okamoto, T., Schlegel, A., Scherer, P. E. & Lisanti, M. P. Caveolins, a family of scaffolding proteins for organizing preassembled signaling complexes at the plasma membrane. J. Biol. Chem. 273, 5419–5422 (1998).
Chambliss, K. L. et al. Estrogen receptor α and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ. Res. 87, E44–E52 (2000).
Razandi, M., Oh, P., Pedram, A., Schnitzer, J. & Levin, E. R. ERs associate with and regulate the production of caveolin: implications for signaling and cellular actions. Mol. Endocrinol. 16, 100–115 (2002).
Schlegel, A., Wang, C. G., Katzenellenbogen, B. S., Pestell, R. G. & Lisanti, M. P. Caveolin-1 potentiates estrogen receptor α (ER α) signaling – caveolin-1 drives ligand-independent nuclear translocation and activation of ER α. J. Biol. Chem. 274, 33551–33556 (1999).
Ikezu, T. et al. Affinity-purification and characterization of caveolins from the brain – differential expression of caveolin-1, -2, and -3 in brain endothelial and astroglial cell types. Brain Res. 804, 177–192 (1998).
Singh, M., Setalo, G., Guan, X. P., Warren, M. & Toran-Allerand, C. D. Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways. J. Neurosci. 19, 1179–1188 (1999).
Kousteni, S. et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104, 719–730 (2001).
Cohen, P. & Frame, S. The renaissance of GSK3. Nature Rev. Mol. Cell Biol. 2, 769–776 (2001).
Campard, P. K. et al. PACAP type I receptor activation promotes cerebellar neuron survival through the cAMP/PKA signaling pathway. DNA Cell Biol. 16, 323–333 (1997).
Honda, K. et al. Nongenomic antiapoptotic signal transduction by estrogen in cultured cortical neurons. J. Neurosci. Res. 64, 466–475 (2001).
Carlstrom, L., Ke, Z. J., Unnerstall, J. R., Cohen, R. S. & Pandey, S. C. Estrogen modulation of the cyclic AMP response element-binding protein pathway – effects of long-term and acute treatments. Neuroendocrinology 74, 227–243 (2001).
Murphy, D. D. & Segal, M. Morphological plasticity of dendritic spines in central neurons is mediated by activation of cAMP response element binding protein. Proc. Natl Acad. Sci. USA 94, 1482–1487 (1997).
Watters, J. J. & Dorsa, D. M. Transcriptional effects of estrogen on neuronal neurotensin gene expression involve cAMP/protein kinase A-dependent signaling mechanisms. J. Neurosci. 18, 6672–6680 (1998).
Zhang, L. et al. Estrogen protects against β-amyloid-induced neurotoxicity in rat hippocampal neurons by activation of Akt. Neuroreport 12, 1919–1923 (2001).
Pozzo-Miller, L. D., Inoue, T. & Murphy, D. D. Estradiol increases spine density and NMDA-dependent Ca2+ transients in spines of CA1 pyramidal neurons from hippocampal slices. J. Neurophysiol. 81, 1404–1411 (1999).
Kelly, M. J., Lagrange, A. H., Wagner, E. J. & Ronnekleiv, O. K. Rapid effects of estrogen to modulate G protein-coupled receptors via activation of protein kinase A and protein kinase C pathways. Steroids 64, 64–75 (1999).
Hayashi, T. et al. Biphasic effect of estrogen on neuronal constitutive nitric oxide synthase via Ca2+–calmodulin dependent mechanism. Biochem. Biophys. Res. Commun. 203, 1013–1019 (1994).
Howard, S. A., Brooke, S. M. & Sapolsky, R.M. Mechanisms of estrogenic protection against gp120-induced neurotoxicity. Exp. Neurol. 168, 385–391 (2001).
Weaver, C. E. et al. Geometry and charge determine pharmacological effects of steroids on N-methyl-d-aspartate receptor-induced Ca2+ accumulation and cell death. J. Pharmacol. Exp. Ther. 293, 747–754 (2000).
Schubert, D. & Piasecki, D. Oxidative glutamate toxicity can be a component of the excitotoxicity cascade. J. Neurosci. 21, 7455–7462 (2001).Shows the importance of oxidative stress in glutamate toxicity.
Weaver, C. E., Parkchung, M., Gibbs, T. T. & Farb, D. H. 17-β-estradiol protects against NMDA-induced excitotoxicity by direct inhibition of NMDA receptors. Brain Res. 761, 338–341 (1997).
Wang, L., Andersson, S., Warner, M. & Gustafsson, J. A. Morphological abnormalities in the brains of estrogen receptor β knockout mice. Proc. Natl Acad. Sci. USA 98, 2792–2796 (2001).
Dubal, D. B. et al. Estrogen receptor α, not β, is a critical link in estradiol-mediated protection against brain injury. Proc. Natl Acad. Sci. USA 98, 1952–1957 (2001).
Forsell, C. et al. Investigations of a CA repeat in the oestrogen receptor β gene in patients with Alzheimer's disease. Eur. J. Hum. Genet. 9, 802–804 (2001).
Sugioka, K., Shimosegawa, Y. & Nakano, M. Estrogens as natural antioxidants of membrane phospholipid peroxidation. FEBS Lett. 210, 37–39 (1987).An early report showing that oestrogen can act as free-radical scavenger in a lipophilic environment.
Moosmann, B. & Behl, C. The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties. Proc. Natl Acad. Sci. USA 96, 8867–8872 (1999).
Behl, C. et al. Neuroprotection against oxidative stress by estrogens: structure–activity relationship. Mol. Pharmacol. 51, 535–541 (1997).The first report to show that the antioxidant neuroprotective activity of 17β-oestradiol is dependent on its phenolic ring structure.
Green, P. S., Bishop, J. & Simpkins, J. W. 17-α-Estradiol exerts neuroprotective effects on SK-N-SH cells. J. Neurosci. 17, 511–515 (1997).
Vedder, H. et al. Estrogen hormones reduce lipid peroxidation in cells and tissues of the central nervous system. J. Neurochem. 72, 2531–2538 (1999).
Vegeto, E., Ciana, P. & Maggi, A. Estrogen and inflammation: hormone generous action spreads to the brain. Mol. Psychiatry 7, 236–238 (2002).
Whitehouse, P. J. et al. Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science 215, 1237–1239 (1982).
Luine, V. N., Khylchevskaya, R. I. & McEwen, B. S. Effect of gonadal steroids on activities of monoamine oxidase and choline acetylase in rat brain. Brain Res. 86, 293–306 (1975).Shows for the first time that oestrogen can affect the metabolism of the neurotransmitter acetylcholine.
Ishunina, T. A. & Swaab, D. F. Increased expression of estrogen receptor α and β in the nucleus basalis of Meynert in Alzheimer's disease. Neurobiol. Aging 22, 417–426 (2001).Shows an upregulation of ER expression in the brains of patients with Alzheimer's disease.
Selkoe, D. J. Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev. 81, 741–766 (2001).
Xu, H. X. et al. Estrogen reduces neuronal generation of Alzheimer β-aymloid peptides. Nature Med. 4, 447–451 (1998).Using clonal neuronal cell lines and primary neurons, this paper reports that oestrogen enhances the non-amyloidogenic processing of the amyloid precursor protein and prevents the formation of Aβ.
Zheng, H. et al. Modulation of Aβ peptides by estrogen in mouse models. J. Neurochem. 80, 191–196 (2002).
Gouras, G. K. et al. Testosterone reduces neuronal secretion of Alzheimer's β-amyloid peptides. Proc. Natl Acad. Sci. USA 97, 1202–1205 (2000).
Goodenough, S., Engert, S. & Behl, C. Testosterone stimulates rapid secretory amyloid precursor protein release from rat hypothalamic cells via the activation of the mitogen-activated protein kinase pathway. Neurosci. Lett. 296, 49–52 (2000).
Sagara, Y. Induction of reactive oxygen species in neurons by haloperidol. J. Neurochem. 71, 1002–1012 (1998).
Chae, H. S. et al. Estrogen attenuates cell death induced by carboxy-terminal fragment of amyloid precursor protein in PC12 through a receptor-dependent pathway. J. Neurosci. Res. 65, 403–407 (2001).
Gollapudi, L. & Oblinger, M. M. Stable transfection of PC12 cells with estrogen receptor α: protective effects of estrogen on cell survival after serum deprivation. J. Neurosci. Res. 56, 99–108 (1999).
Acknowledgements
I thank S. Goodenough, N. Bayatti and D. Manthey for fruitful discussions. The work of my laboratory is supported in part by grants from the Deutsche Forschungsgemeinschaft and the European Union.
Author information
Authors and Affiliations
Related links
Related links
DATABASES
LocusLink
Medscape DrugInfo
OMIM
FURTHER INFORMATION
Encyclopedia of Life Sciences
Glossary
- REPERFUSION DAMAGE
-
The tissue damage that occurs after an episode of ischaemia, not as a result of lack of blood, but as a consequence of the return of blood to the affected region.
- HILUS
-
A subdivision of the hippocampus that is rich in interneurons. It is located between the CA3 region and the dentate gyrus.
- NUCLEUS BASALIS OF MEYNERT
-
A telencephalic structure that provides most of the acetylcholine to the cerebral cortex.
- INVERTED REPEAT
-
A nucleotide sequence that is found at two sites on the same DNA segment, but with opposite orientations.
- PALINDROMIC SEQUENCE
-
A sequence of nucleotides that reads the same regardless of direction.
- NEUROFILAMENT
-
A type of intermediate filament that is found only in neurons and serves as a cytoskeletal element that supports the axonal cytoplasm.
- SH DOMAINS
-
Src-homology domains are involved in interactions with phosphorylated tyrosine residues on other proteins (SH2 domains) or with proline-rich sections of other proteins (SH3 domains).
- FK506
-
A metabolic product of the fungus Streptomyces tsukabaensis that is commonly used as an immunosupressant agent. FK506 has a binding protein that, when bound to the drug, inhibits the phosphatase calcineurin.
- CAVEOLAE
-
Specialized rafts that contain the protein caveolin and form a flask-shaped, cholesterol-rich invagination of the plasma membrane, which might mediate the uptake of some extracellular materials and are probably involved in cell signalling.
- EXPRESSION PROFILING
-
The use of DNA microarrays to determine the expression level of thousands of genes simultaneously.
Rights and permissions
About this article
Cite this article
Behl, C. Oestrogen as a neuroprotective hormone. Nat Rev Neurosci 3, 433–442 (2002). https://doi.org/10.1038/nrn846
Issue Date:
DOI: https://doi.org/10.1038/nrn846
This article is cited by
-
Sex and the Estrous-Cycle Phase Influence the Expression of G Protein-Coupled Estrogen Receptor 1 (GPER) in Schizophrenia: Translational Evidence for a New Target
Molecular Neurobiology (2023)
-
Associations between catechol-O-methyltransferase (COMT) genotypes at rs4818 and rs4680 and gene expression in human dorsolateral prefrontal cortex
Experimental Brain Research (2020)
-
The regulatory role of Toll-like receptors after ischemic stroke: neurosteroids as TLR modulators with the focus on TLR2/4
Cellular and Molecular Life Sciences (2019)
-
Loss of Angelman Syndrome Protein E6AP Disrupts a Novel Antagonistic Estrogen-Retinoic Acid Transcriptional Crosstalk in Neurons
Molecular Neurobiology (2018)
-
Evaluating the neuroprotective effect of 17β-estradiol in rodent models of oxidative retinopathies
Documenta Ophthalmologica (2018)