Key Points
-
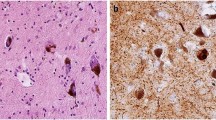
Notable heterogeneity exists in the neuropathological substrates that underlie dementia in the setting of Parkinson's disease dementia (PDD). Nevertheless, the presumptive caudal-to-rostral spread of Lewy body and neurite pathology from the lower brainstem to telencephalic regions, which culminates in a heavy burden of this α-synuclein (α-syn) pathology in limbic and neocortical structures, is the most characteristic pathological finding in most PDD cases.
-
Up to 50% of patients with PDD can have sufficient amyloid-β (Aβ) plaque and tau neurofibrillary tangle (NFT) pathology for the diagnosis of a second neurodegenerative dementia — that is, Alzheimer's disease (AD) — and this co-morbid pathology is more common in PDD than PD.
-
Tau NFT and Aβ plaque pathology may act synergistically with Lewy body and neurite pathology to confer a worse prognosis and a higher burden of cortical Lewy body and neurite pathology in PDD.
-
Clinical phenotypes of PD may help to identify neuropathological subtypes of PD that exhibit differing propensities for developing dementia. For example, non-tremor-dominant or postural gait instability PD phenotypes may often be associated with greater Aβ plaque pathology and shorter times to dementia in patients with PDD than in patients with PD exhibiting less co-morbid AD neuropathology and a tremor-dominant phenotype.
-
Genetic variations may contribute to the heterogeneity in the neuropathology and the time-of-onset of dementia in PD. For example, the apolipoprotein E (APOE) ε4 genotype may increase both Lewy body and neurite pathology and AD neuropathology and result in an increased risk of dementia in PD. Moreover, heterozygous mutations in β-glucocerebrosidase (GBA) may increase the severity of Lewy body and neurite patholgy in 'pure' synucleinopathies.
-
Further biomarker and detailed clinicopathological correlation studies of prospective patients will help to further elucidate the inter-relationships of AD and α-syn pathology in PD and the development of dementia in patients with PD. These discoveries will be crucial in the development of meaningful disease-modifying therapies for PDD.
Abstract
Dementia is increasingly being recognized in cases of Parkinson's disease (PD); such cases are termed PD dementia (PDD). The spread of fibrillar α-synuclein (α-syn) pathology from the brainstem to limbic and neocortical structures seems to be the strongest neuropathological correlate of emerging dementia in PD. In addition, up to 50% of patients with PDD also develop sufficient numbers of amyloid-β plaques and tau-containing neurofibrillary tangles for a secondary diagnosis of Alzheimer's disease, and these pathologies may act synergistically with α-syn pathology to confer a worse prognosis. An understanding of the relationships between these three distinct pathologies and their resultant clinical phenotypes is crucial for the development of effective disease-modifying treatments for PD and PDD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Goedert, M., Spillantini, M. G., Del Tredici, K. & Braak, H. 100 years of Lewy pathology. Nature Rev. Neurol. 9, 13–24 (2012).
Hely, M. A., Reid, W. G., Adena, M. A., Halliday, G. M. & Morris, J. G. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov. Disord. 23, 837–844 (2008).
Aarsland, D., Andersen, K., Larsen, J. P., Lolk, A. & Kragh-Sorensen, P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch. Neurol. 60, 387–392 (2003).
Kempster, P. A., O'Sullivan, S. S., Holton, J. L., Revesz, T. & Lees, A. J. Relationships between age and late progression of Parkinson's disease: a clinico-pathological study. Brain 133, 1755–1762 (2010).
Emre, M. et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov. Disord. 22, 1689–1707 (2007). The Movement Disorder Society Task Force criteria for PDD.
Halliday, G., Hely, M., Reid, W. & Morris, J. The progression of pathology in longitudinally followed patients with Parkinson's disease. Acta Neuropathol. 115, 409–415 (2008). This is one of the largest prospectively followed PD autopsy series; it found that 80% of patients with PD develop dementia after 20 years.
Levy, G. et al. Combined effect of age and severity on the risk of dementia in Parkinson's disease. Ann. Neurol. 51, 722–729 (2002).
Williams-Gray, C. H. et al. The distinct cognitive syndromes of Parkinson's disease: 5 year follow-up of the CamPaIGN cohort. Brain 132, 2958–2969 (2009).
Muslimovic, D., Post, B., Speelman, J. D. & Schmand, B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology 65, 1239–1245 (2005).
Rosenthal, E. et al. Association between cognition and function in patients with Parkinson disease with and without dementia. Mov. Disord. 25, 1170–1176 (2010).
Lo, R. Y. et al. Clinical features in early Parkinson disease and survival. Arch. Neurol. 66, 1353–1358 (2009).
Litvan, I. et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov. Disord. 27, 349–356 (2012). The Movement Disorder Society Task Force criteria for PD-MCI.
Grossman, M. et al. Difficulty processing temporary syntactic ambiguities in Lewy body spectrum disorder. Brain Lang. 120, 52–60 (2012).
Lippa, C. F. et al. DLB and PDD boundary issues: diagnosis, treatment, molecular pathology, and biomarkers. Neurology 68, 812–819 (2007). An in-depth discussion of the clinicopathological overlap between PDD and DLB.
Galvin, J. E., Pollack, J. & Morris, J. C. Clinical phenotype of Parkinson disease dementia. Neurology 67, 1605–1611 (2006).
McKeith, I. G. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J. Alzheimers Dis. 9, 417–423 (2006). In this paper, the DLB Consortium outlines its clinical and neuropathological diagnostic criteria for DLB.
Weintraub, D. Dopamine and impulse control disorders in Parkinson's disease. Ann. Neurol. 64 (Suppl. 2), 93–100 (2008).
Weintraub, D., Papay, K., Siderowf, A. & Parkinson's Progression Markers Initiative. Screening for impulse control symptoms in patients with de novo Parkinson disease: a case-control study. Neurology 80, 176–180 (2013).
Schrag, A., Ben-Shlomo, Y., Brown, R., Marsden, C. D. & Quinn, N. Young-onset Parkinson's disease revisited — clinical features, natural history, and mortality. Mov. Disord. 13, 885–894 (1998).
Aarsland, D. et al. The effect of age of onset of PD on risk of dementia. J. Neurol. 254, 38–45 (2007).
Braak, H., Rub, U., Jansen Steur, E. N., Del Tredici, K. & de Vos, R. A. Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology 64, 1404–1410 (2005).
Levy, G. et al. Motor impairment in PD: relationship to incident dementia and age. Neurology 55, 539–544 (2000).
Jankovic, J. et al. Variable expression of Parkinson's disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology 40, 1529–1534 (1990).
Levy, G. et al. Memory and executive function impairment predict dementia in Parkinson's disease. Mov. Disord. 17, 1221–1226 (2002).
Polymeropoulos, M. H. et al. Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science 276, 2045–2047 (1997). This study made the landmark discovery of SNCA mutations in PD.
Poulopoulos, M., Levy, O. A. & Alcalay, R. N. The neuropathology of genetic Parkinson's disease. Mov. Disord. 27, 831–842 (2012).
Singleton, A. B. et al. α-synuclein locus triplication causes Parkinson's disease. Science 302, 841 (2003).
Conway, K. A., Harper, J. D. & Lansbury, P. T. Accelerated in vitro fibril formation by a mutant α-synuclein linked to early-onset Parkinson disease. Nature Med. 4, 1318–1320 (1998).
Spillantini, M. G. et al. α-Synuclein in Lewy bodies. Nature 388, 839–840 (1997). This study made the landmark discovery of α-syn as the major component of Lewy pathology.
Giasson, B. I. et al. Neuronal α-synucleinopathy with severe movement disorder in mice expressing A53T human α-synuclein. Neuron 34, 521–533 (2002).
Lim, Y. et al. α-Syn suppression reverses synaptic and memory defects in a mouse model of dementia with Lewy bodies. J. Neurosci. 31, 10076–10087 (2011).
Magen, I. & Chesselet, M. F. Genetic mouse models of Parkinson's disease: the state of the art. Prog. Brain Res. 184, 53–87 (2010).
Braak, H. et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging 24, 197–211 (2003).
Del Tredici, K., Rub, U., De Vos, R. A., Bohl, J. R. & Braak, H. Where does parkinson disease pathology begin in the brain? J. Neuropathol. Exp. Neurol. 61, 413–426 (2002).
Dickson, D. W., Uchikado, H., Fujishiro, H. & Tsuboi, Y. Evidence in favor of Braak staging of Parkinson's disease. Mov. Disord. 25 (Suppl. 1), 78–82 (2010).
Jellinger, K. A. Lewy body-related α-synucleinopathy in the aged human brain. J. Neural Transm. 111, 1219–1235 (2004).
Parkkinen, L., Pirttila, T. & Alafuzoff, I. Applicability of current staging/categorization of α-synuclein pathology and their clinical relevance. Acta Neuropathol. 115, 399–407 (2008).
Beach, T. G. et al. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 117, 613–634 (2009).
Mikolaenko, I. et al. α-synuclein lesions in normal aging, Parkinson disease, and Alzheimer disease: evidence from the Baltimore Longitudinal Study of Aging (BLSA). J. Neuropathol. Exp. Neurol. 64, 156–162 (2005).
Parkkinen, L., Kauppinen, T., Pirttila, T., Autere, J. M. & Alafuzoff, I. α-synuclein pathology does not predict extrapyramidal symptoms or dementia. Ann. Neurol. 57, 82–91 (2005).
Dickson, D. W. et al. Evidence that incidental Lewy body disease is pre-symptomatic Parkinson's disease. Acta Neuropathol. 115, 437–444 (2008).
Saito, Y. et al. Accumulation of phosphorylated α-synuclein in aging human brain. J. Neuropathol. Exp. Neurol. 62, 644–654 (2003).
Wakabayashi, K. et al. The Lewy body in Parkinson's disease and related neurodegenerative disorders. Mol. Neurobiol. 47, 495–508 (2013).
Milber, J. M. et al. Lewy pathology is not the first sign of degeneration in vulnerable neurons in Parkinson disease. Neurology 79, 2307–2314 (2012).
Parkkinen, L. et al. Disentangling the relationship between Lewy bodies and nigral neuronal loss in Parkinson's disease. J. Park Dis. 1, 277–286 (2011).
Braak, H. et al. Pathology associated with sporadic Parkinson's disease — where does it end? J. Neural Transm. Suppl. 70, 89–97 (2006).
Hamilton, R. L. Lewy bodies in Alzheimer's disease: a neuropathological review of 145 cases using α-synuclein immunohistochemistry. Brain Pathol. 10, 378–384 (2000).
Lippa, C. F. et al. Lewy bodies contain altered α-synuclein in brains of many familial Alzheimer's disease patients with mutations in presenilin and amyloid precursor protein genes. Am. J. Pathol. 153, 1365–1370 (1998).
Iseki, E. Dementia with Lewy bodies: reclassification of pathological subtypes and boundary with Parkinson's disease or Alzheimer's disease. Neuropathology 24, 72–78 (2004).
Leverenz, J. B. et al. Empiric refinement of the pathologic assessment of Lewy-related pathology in the dementia patient. Brain Pathol. 18, 220–224 (2008).
Jellinger, K. A. A critical reappraisal of current staging of Lewy-related pathology in human brain. Acta Neuropathol. 116, 1–16 (2008).
Li, J. Y. et al. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nature Med. 14, 501–503 (2008).
Kordower, J. H., Chu, Y., Hauser, R. A., Freeman, T. B. & Olanow, C. W. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nature Med. 14, 504–506 (2008).
Kordower, J. H., Chu, Y., Hauser, R. A., Olanow, C. W. & Freeman, T. B. Transplanted dopaminergic neurons develop PD pathologic changes: a second case report. Mov. Disord. 23, 2303–2306 (2008).
Luk, K. C. et al. Exogenous α-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc. Natl Acad. Sci. USA 106, 20051–20056 (2009).
Volpicelli-Daley, L. A. et al. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 72, 57–71 (2011).
Luk, K. C. et al. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J. Exp. Med. 209, 975–986 (2012).
Luk, K. C. et al. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338, 949–953 (2012). This study made the landmark discovery of how the transmission of α-syn fibrils alone recapitulates human disease in wild-type animals.
Lee, H. J. et al. Assembly-dependent endocytosis and clearance of extracellular α-synuclein. Int. J. Biochem. Cell Biol. 40, 1835–1849 (2008).
Desplats, P. et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of α-synuclein. Proc. Natl Acad. Sci. USA 106, 13010–13015 (2009).
Mougenot, A. L. et al. Prion-like acceleration of a synucleinopathy in a transgenic mouse model. Neurobiol. Aging 33, 2225–2228 (2012).
Masuda-Suzukake, M. et al. Prion-like spreading of pathological α-synuclein in brain. Brain 136, 1128–1138 (2013).
Steiner, J. A., Angot, E. & Brundin, P. A deadly spread: cellular mechanisms of α-synuclein transfer. Cell Death Differ. 18, 1425–1433 (2011).
Olanow, C. W. & Brundin, P. Parkinson's disease and alpha synuclein: is Parkinson's disease a prion-like disorder? Mov. Disord. 28, 31–40 (2013). A timely review of PD model transmission studies.
Lee, V. M. & Trojanowski, J. Q. Mechanisms of Parkinson's disease linked to pathological α-synuclein: new targets for drug discovery. Neuron 52, 33–38 (2006).
Irwin, D. J. et al. Evaluation of potential infectivity of Alzheimer's and Parkinson's disease proteins in recipients of cadaver-derived human growth hormone. JAMA Neurol. 70, 462–468 (2013).
Hurtig, H. I. et al. Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson's disease. Neurology 54, 1916–1921 (2000).
Duda, J. E., Giasson, B. I., Mabon, M. E., Lee, V. M. & Trojanowski, J. Q. Novel antibodies to synuclein show abundant striatal pathology in Lewy body diseases. Ann. Neurol. 52, 205–210 (2002).
Apaydin, H., Ahlskog, J. E., Parisi, J. E., Boeve, B. F. & Dickson, D. W. Parkinson disease neuropathology: later-developing dementia and loss of the levodopa response. Arch. Neurol. 59, 102–112 (2002).
Irwin, D. J. et al. Neuropathologic substrates of Parkinson disease dementia. Ann. Neurol. 72, 587–598 (2012). A large autopsy cohort involving multivariate analysis of multiple clinical, genetic and neuropathological variables that implicate Lewy body and neurite pathology as the strongest correlate of PDD.
Compta, Y. et al. Lewy- and Alzheimer-type pathologies in Parkinson's disease dementia: which is more important? Brain. 134, 1493–1505 (2011).
Tsuboi, Y., Uchikado, H. & Dickson, D. W. Neuropathology of Parkinson's disease dementia and dementia with Lewy bodies with reference to striatal pathology. Parkinsonism Relat. Disord. 13 (Suppl. 3), 221–224 (2007).
Jellinger, K. A. & Attems, J. Prevalence and impact of vascular and Alzheimer pathologies in Lewy body disease. Acta Neuropathol. 115, 427–436 (2008).
Harding, A. J. & Halliday, G. M. Cortical Lewy body pathology in the diagnosis of dementia. Acta Neuropathol. 102, 355–363 (2001).
Kovari, E. et al. Lewy body densities in the entorhinal and anterior cingulate cortex predict cognitive deficits in Parkinson's disease. Acta Neuropathol. 106, 83–88 (2003).
Mattila, P. M., Rinne, J. O., Helenius, H., Dickson, D. W. & Roytta, M. α-synuclein-immunoreactive cortical Lewy bodies are associated with cognitive impairment in Parkinson's disease. Acta Neuropathol. 100, 285–290 (2000).
Pletnikova, O. et al. Aβ deposition is associated with enhanced cortical α-synuclein lesions in Lewy body diseases. Neurobiol. Aging 26, 1183–1192 (2005).
Ballard, C. et al. Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology 67, 1931–1934 (2006).
Perry, E. K. et al. Cholinergic correlates of cognitive impairment in Parkinson's disease: comparisons with Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry 48, 413–421 (1985).
Whitehouse, P. J., Hedreen, J. C., White, C. L., & Price, D. L. Basal forebrain neurons in the dementia of Parkinson disease. Ann. Neurol. 13, 243–248 (1983).
Kalaitzakis, M. E., Graeber, M. B., Gentleman, S. M. & Pearce, R. K. Striatal β-amyloid deposition in Parkinson disease with dementia. J. Neuropathol. Exp. Neurol. 67, 155–161 (2008).
Jellinger, K. A. Morphological substrates of parkinsonism with and without dementia: a retrospective clinico-pathological study. J. Neural. Transm. Suppl. 72, 91–104 (2007).
Jellinger, K. A., Seppi, K., Wenning, G. K. & Poewe, W. Impact of coexistent Alzheimer pathology on the natural history of Parkinson's disease. J. Neural Transm. 109, 329–339 (2002).
Kotzbauer, P. T. et al. Pathologic accumulation of α-synuclein and Aβ in Parkinson disease patients with dementia. Arch. Neurol. 69, 1326–1331 (2012).
Hughes, A. J., Daniel, S. E., Blankson, S. & Lees, A. J. A clinicopathologic study of 100 cases of Parkinson's disease. Arch. Neurol. 50, 140–148 (1993).
Sabbagh, M. N. et al. Parkinson disease with dementia: comparing patients with and without Alzheimer pathology. Alzheimer Dis. Assoc. Disord. 23, 295–297 (2009).
Lashley, T. et al. Cortical α-synuclein load is associated with amyloid-β plaque burden in a subset of Parkinson's disease patients. Acta Neuropathol. 115, 417–425 (2008).
Masliah, E. et al. β-amyloid peptides enhance α-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer's disease and Parkinson's disease. Proc. Natl Acad. Sci. USA 98, 12245–12250 (2001).
Clinton, L. K., Blurton-Jones, M., Myczek, K., Trojanowski, J. Q. & LaFerla, F. M. Synergistic interactions between Aβ, tau, and α-synuclein: acceleration of neuropathology and cognitive decline. J. Neurosci. 30, 7281–7289 (2010).
Duda, J. E. et al. Concurrence of α-synuclein and tau brain pathology in the Contursi kindred. Acta Neuropathol. 104, 7–11 (2002).
Lee, V. M., Giasson, B. I. & Trojanowski, J. Q. More than just two peas in a pod: common amyloidogenic properties of tau and α-synuclein in neurodegenerative diseases. Trends Neurosci. 27, 129–134 (2004).
Giasson, B. I. et al. Initiation and synergistic fibrillization of tau and α-synuclein. Science 300, 636–640 (2003).
Jellinger, K. A., Wenning, G. K. & Seppi, K. Predictors of survival in dementia with lewy bodies and Parkinson dementia. Neurodegener. Dis. 4, 428–430 (2007).
Sabbagh, M. N. et al. Correlation of clinical features with argyrophilic grains at autopsy. Alzheimer Dis. Assoc. Disord. 23, 229–233 (2009).
Nakashima-Yasuda, H. et al. Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol. 114, 221–229 (2007).
Colosimo, C., Hughes, A. J., Kilford, L. & Lees, A. J. Lewy body cortical involvement may not always predict dementia in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 74, 852–856 (2003).
Richard, I. H., Papka, M., Rubio, A. & Kurlan, R. Parkinson's disease and dementia with Lewy bodies: one disease or two? Mov. Disord. 17, 1161–1165 (2002).
Jellinger, K. A. & Attems, J. Does striatal pathology distinguish Parkinson disease with dementia and dementia with Lewy bodies? Acta Neuropathol. 112, 253–260 (2006).
Halliday, G. M., Song, Y. J. & Harding, A. J. Striatal β-amyloid in dementia with Lewy bodies but not Parkinson's disease. J. Neural Transm. 118, 713–719 (2011).
Merdes, A. R. et al. Influence of Alzheimer pathology on clinical diagnostic accuracy in dementia with Lewy bodies. Neurology 60, 1586–1590 (2003).
Verghese, J., Crystal, H. A., Dickson, D. W. & Lipton, R. B. Validity of clinical criteria for the diagnosis of dementia with Lewy bodies. Neurology 53, 1974–1982 (1999).
Litvan, I. et al. Accuracy of the clinical diagnoses of Lewy body disease, Parkinson disease, and dementia with Lewy bodies: a clinicopathologic study. Arch. Neurol. 55, 969–978 (1998).
Nelson, P. T. et al. Low sensitivity in clinical diagnoses of dementia with Lewy bodies. J. Neurol. 257, 359–366 (2010).
Kraybill, M. L. et al. Cognitive differences in dementia patients with autopsy-verified AD, Lewy body pathology, or both. Neurology 64, 2069–2073 (2005).
van Rooden, S. M. et al. The identification of Parkinson's disease subtypes using cluster analysis: a systematic review. Mov. Disord. 25, 969–978 (2010).
Selikhova, M. et al. A clinico-pathological study of subtypes in Parkinson's disease. Brain 132, 2947–2957 (2009).
Alves, G., Larsen, J. P., Emre, M., Wentzel-Larsen, T. & Aarsland, D. Changes in motor subtype and risk for incident dementia in Parkinson's disease. Mov. Disord. 21, 1123–1130 (2006).
Prikrylova Vranova, H. et al. CSF markers of neurodegeneration in Parkinson's disease. J. Neural Transm. 117, 1177–1181 (2010).
Alves, G. et al. Cerebrospinal fluid amyloid-β and phenotypic heterogeneity in de novo Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 85, 537–543 (2012).
Kang, J. H. et al. Association of cerebrospinal fluid Aβ1-42, t-tau, p-tau181 and α-synuclein levels with clinical 1 features of early drug naïve Parkinson's disease patients. JAMA Neurol. (in the press).
Müller, M. L. et al. β-amyloid and postural instability and gait difficulty in Parkinson's disease at risk for dementia. Mov. Disord. 28, 296–301 (2012).
Halliday, G. M., Holton, J. L., Revesz, T. & Dickson, D. W. Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol. 122, 187–204 (2011).
Svenningsson, P., Westman, E., Ballard, C. & Aarsland, D. Cognitive impairment in patients with Parkinson's disease: diagnosis, biomarkers, and treatment. Lancet Neurol. 11, 697–707 (2012).
Sidransky, E. et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N. Engl. J. Med. 361, 1651–1661 (2009).
Tsuang, D. et al. GBA mutations increase risk for Lewy body disease with and without Alzheimer disease pathology. Neurology 79, 1944–1950 (2012).
Neumann, J. et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson's disease. Brain 132, 1783–1794 (2009).
Nalls, M. A. et al. A multicenter study of glucocerebrosidase mutations in dementia with lewy bodies. JAMA Neurol. 70, 727–735 (2013).
Clark, L. N. et al. Association of glucocerebrosidase mutations with dementia with lewy bodies. Arch. Neurol. 66, 578–583 (2009).
Morley, J. F. et al. Genetic influences on cognitive decline in Parkinson's disease. Mov. Disord. 27, 512–518 (2012).
Tsuang, D. et al. APOE ε4 increases risk for dementia in pure synucleinopathies. JAMA Neurol. 70, 223–228 (2013).
Williams-Gray, C. H. et al. Apolipoprotein E genotype as a risk factor for susceptibility to and dementia in Parkinson's disease. J. Neurol. 256, 493–498 (2009).
Wakabayashi, K. et al. Apolipoprotein E ε4 allele and progression of cortical Lewy body pathology in Parkinson's disease. Acta Neuropathol. 95, 450–454 (1998).
Siderowf, A. et al. CSF amyloid β1–42 predicts cognitive decline in Parkinson disease. Neurology 75, 1055–1061 (2010).
Baker, M. et al. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum. Mol. Genet. 8, 711–715 (1999).
Zabetian, C. P. et al. Association analysis of MAPT H1 haplotype and subhaplotypes in Parkinson's disease. Ann. Neurol. 62, 137–144 (2007).
Goris, A. et al. Tau and α-synuclein in susceptibility to, and dementia in, Parkinson's disease. Ann. Neurol. 62, 145–153 (2007).
Compta, Y. et al. High cerebrospinal tau levels are associated with the rs242557 tau gene variant and low cerebrospinal β-amyloid in Parkinson disease. Neurosci. Lett. 487, 169–173 (2011).
Chen-Plotkin, A. S. et al. Plasma epidermal growth factor levels predict cognitive decline in Parkinson disease. Ann. Neurol. 69, 655–663 (2011).
Pellecchia, M. T. et al. Serum epidermal growth factor predicts cognitive functions in early, drug-naive Parkinson's disease patients. J. Neurol. 260, 438–444 (2012).
Compta, Y. et al. Cerebrospinal tau, phospho-tau, and β-amyloid and neuropsychological functions in Parkinson's disease. Mov. Disord. 24, 2203–2210 (2009).
Hall, S. et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. JAMA Neurol. 69, 1445–1452 (2012).
Alves, G. et al. CSF amyloid-β and tau proteins, and cognitive performance, in early and untreated Parkinson's disease: the Norwegian ParkWest study. J. Neurol. Neurosurg. Psychiatry 81, 1080–1086 (2010).
Montine, T. J. et al. CSF Aβ42 and tau in Parkinson's disease with cognitive impairment. Mov. Disord. 25, 2682–2685 (2010).
Leverenz, J. B. et al. Cerebrospinal fluid biomarkers and cognitive performance in non-demented patients with Parkinson's disease. Parkinsonism Relat. Disord. 17, 61–64 (2011).
Mollenhauer, B. et al. Direct quantification of CSF α-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp. Neurol. 213, 315–325 (2008).
Wang, Y. et al. Phosphorylated α-synuclein in Parkinson's disease. Sci. Transl. Med. 4, 121ra20 (2012).
Tokuda, T. et al. Detection of elevated levels of α-synuclein oligomers in CSF from patients with Parkinson disease. Neurology 75, 1766–1772 (2010).
Hong, Z. et al. DJ-1 and α-synuclein in human cerebrospinal fluid as biomarkers of Parkinson's disease. Brain 133, 713–726 (2010).
Shi, M. et al. Cerebrospinal fluid biomarkers for Parkinson disease diagnosis and progression. Ann. Neurol. 69, 570–580 (2011).
Compta, Y. et al. Grey matter volume correlates of cerebrospinal markers of Alzheimer-pathology in Parkinson's disease and related dementia. Parkinsonism Relat. Disord. 18, 941–947 (2012).
Weintraub, D. et al. Alzheimer's disease pattern of brain atrophy predicts cognitive decline in Parkinson's disease. Brain 135, 170–180 (2011).
Gomperts, S. N. et al. Amyloid is linked to cognitive decline in patients with Parkinson disease without dementia. Neurology 80, 85–91 (2013).
Petrou, M. et al. Aβ-amyloid deposition in patients with Parkinson disease at risk for development of dementia. Neurology 79, 1161–1167 (2012).
Bohnen, N. I. et al. Cerebral glucose metabolic features of Parkinson disease and incident dementia: longitudinal study. J. Nucl. Med. 52, 848–855 (2011).
Beyer, M. K. et al. Cerebrospinal fluid Aβ levels correlate with structural brain changes in Parkinson's disease. Mov. Disord. 28, 302–310 (2013).
Marek, K. et al. The Parkinson Progression Marker Initiative (PPMI). Prog. Neurobiol. 95, 629–635 (2011).
Rolinski, M., Fox, C., Maidment, I. & McShane, R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson's disease dementia and cognitive impairment in Parkinson's disease. Cochrane Database Syst. Rev. 3, CD006504 (2012).
Seppi, K. et al. The movement disorder society evidence-based medicine review update: treatments for the non-motor symptoms of Parkinson's disease. Mov. Disord. 26 (Suppl. 3), 42–80 (2011).
Lashuel, H. A., Overk, C. R., Oueslati, A. & Masliah, E. The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nature Rev. Neurosci. 14, 38–48 (2013). A recent review highlighting the pathophysiology of α-syn toxicity in PD and potential therapeutic strategies.
Valera, E. & Masliah, E. Immunotherapy for neurodegenerative diseases: focus on α-synucleinopathies. Pharmacol. Ther. 138, 311–322 (2013).
Sperling, R. A. et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 7, 280–292 (2011).
Montine, T. J. et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 123, 1–11 (2012).
Ward, C. D. & Gibb, W. R. Research diagnostic criteria for Parkinson's disease. Adv. Neurol. 53, 245–249 (1990).
Prusiner, S. B. Novel proteinaceous infectious particles cause scrapie. Science 216, 136–144 (1982).
Kordower, J. H. & Brundin, P. Propagation of host disease to grafted neurons: accumulating evidence. Exp. Neurol. 220, 224–225 (2009).
Brown, P. et al. Human spongiform encephalopathy: the National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann. Neurol. 35, 513–529 (1994).
Brown, P., Gajdusek, D. C., Gibbs, C. J. Jr & Asher, D. M. Potential epidemic of Creutzfeldt–Jakob disease from human growth hormone therapy. N. Engl. J. Med. 313, 728–731 (1985).
Acknowledgements
We thank the patients and their families who have contributed to the research reviewed here, which has led to meaningful developments in our understanding of Parkinson's disease and related disorders. Funding for our research was provided by the US National Institutes of Health grants P30 AG10124, AG17586, P50 NS53488 and T32-AG000255.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
PowerPoint slides
Glossary
- Executive functioning
-
Abilities in mental flexibility, planning and working memory that are mediated by striatal–frontal networks.
- Semantic memory
-
Memory for the meaning and context of objects and concepts that is mediated by the temporal lobe and its connections throughout the neocortex.
- Mild cognitive impairment
-
(MCI). MCI comprises subjective cognitive complaints with objective findings of cognitive impairment in one or more cognitive domains that does not interfere with the patient's ability to perform tasks of daily living. MCI is thought to represent a prodromal state to Alzheimer's disease and other dementias. Recently, clinical criteria have been defined for MCI in the setting of Parkinson's disease.
- Bradykinesia
-
Symptoms of slowed movement seen in Parkinson's disease and other disorders involving nigral–striatal dopaminergic pathways.
- Constructional praxis
-
The ability to draw or copy a figure (such as clock-drawing or drawing intersecting pentagons), which relies on attention, planning and organization skills (executive function) and visuospatial perceptual abilities.
- Verbal memory
-
Short-term memory for words and verbal information that is partially mediated by language function (for example, memory for words tested through a list-learning task).
- Transmission
-
The spread of a pathological protein in an altered conformation (for example, PrPSc) between neurons within an individual; transmission does not necessarily imply that the disease protein is infectious (that is, it can be spread between individuals).
- Amyloid fibrils
-
Insoluble filamentous structures composed of polymerized protein monomers with notable β-sheet conformation, which can be detected with amyloid-binding dyes (for example, thioflavin S).
- Cognitive reserve
-
This refers to the notion of relative resistance to clinical symptoms of neurodegeneration and other CNS insults that is thought to be mediated by neuroplasticity or an ability to recruit additional brain networks to compensate for the disease state; such plasticity may be influenced by education or other environmental or genetic factors.
- Cerebrovascular disease
-
(CVD). Damage to intracerebral blood vessels from atherosclerosis and lipohyalinosis, which are caused by systemic cardiovascular risk factors (for example, hypertension, diabetes and hyperlipidaemia) and result in ischaemic damage to the brain parenchyma (for example, lacunar infarcts).
- Amyloid angiopathy
-
A form of cerebral vasculopathy that is caused by fibrillar amyloid-β deposition in blood vessel walls.
- Tauopathies
-
A family of neurodegenerative disease proteinopathies that are characterized by inclusions composed primarily of the microtubule-associated protein tau.
Rights and permissions
About this article
Cite this article
Irwin, D., Lee, VY. & Trojanowski, J. Parkinson's disease dementia: convergence of α-synuclein, tau and amyloid-β pathologies. Nat Rev Neurosci 14, 626–636 (2013). https://doi.org/10.1038/nrn3549
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3549