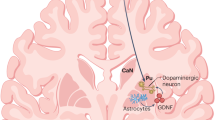
Abstract
Neuropathological changes in Parkinson's disease progress slowly and spread according to a characteristic pattern. Recent papers have shed light on this progression of pathology by examining the fate of neurons grafted into the brains of patients with Parkinson's disease. Two of these studies demonstrate that grafted healthy neurons can gradually develop the same pathology as host neurons in the diseased brains. According to these studies, implanted neurons developed α-synuclein- and ubiquitin-positive Lewy bodies more than a decade after transplantation. We discuss the possible underlying mechanisms and their implications for how pathology spreads in Parkinson's disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Poewe, W. Non-motor symptoms in Parkinson's disease. Eur. J. Neurol. 15 (Suppl. 1), 14–20 (2008).
Braak, H. et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging 24, 197–211 (2003).
Ince, P. G., Clark, B., Holton, J. L., Revesz, T. & Wharton, S. in Greenfield's Neuropathology (eds Ellison, D. W., Louis, D. N. & Love, S.) 889–1030 (Arnold, London, 2008).
Kordower, J. H., Chu, Y., Hauser, R. A., Freeman, T. B. & Olanow, C. W. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nature Med. 14, 504–506 (2008).
Li, J. Y. et al. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nature Med. 14, 501–503 (2008).
Mendez, I. et al. Dopamine neurons implanted into people with Parkinson's disease survive without pathology for 14 years. Nature Med. 14, 507–509 (2008).
Uryu, K. et al. Convergence of heat shock protein 90 with ubiquitin in filamentous α-synuclein inclusions of α-synucleinopathies. Am. J. Pathol. 168, 947–961 (2006).
Uversky, V. N. Neuropathology, biochemistry, and biophysics of α-synuclein aggregation. J. Neurochem. 103, 17–37 (2007).
Halliday, G. M. et al. α-synuclein redistributes to neuromelanin lipid in the substantia nigra early in Parkinson's disease. Brain 128, 2654–2664 (2005).
Chu, Y. & Kordower, J. H. Age-associated increases of α-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: is this the target for Parkinson's disease? Neurobiol. Dis. 25, 134–149 (2007).
Greffard, S. et al. A stable proportion of Lewy body bearing neurons in the substantia nigra suggests a model in which the Lewy body causes neuronal death. Neurobiol. Aging 3 May 2008 (doi:10.1016/j.neurobiolaging.2008.03.015).
Goldmann Gross, R., Siderowf, A. & Hurtig, H. I. Cognitive impairment in Parkinson's disease and dementia with lewy bodies: a spectrum of disease. Neurosignals 16, 24–34 (2008).
McKeith, I. G. et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65, 1863–1872 (2005).
Dickson, D. W. et al. Evidence that incidental Lewy body disease is pre-symptomatic Parkinson's disease. Acta Neuropathol. 115, 437–444 (2008).
Farrer, M. et al. Comparison of kindreds with parkinsonism and α-synuclein genomic multiplications. Ann. Neurol. 55, 174–179 (2004).
Fuchs, J. et al. Phenotypic variation in a large Swedish pedigree due to SNCA duplication and triplication. Neurology 68, 916–922 (2007).
Goedert, M., Jakes, R. & Spillantini, M. G. Alpha-synuclein and the Lewy body. NeuroScience News 1, 2–7 (1998).
Wood-Kaczmar, A., Gandhi, S. & Wood, N. W. Understanding the molecular causes of Parkinson's disease. Trends Mol. Med. 12, 521–528 (2006).
Schiesling, C., Kieper, N., Seidel, K. & Kruger, R. Review: Familial Parkinson's disease — genetics, clinical phenotype and neuropathology in relation to the common sporadic form of the disease. Neuropathol. Appl. Neurobiol. 34, 255–271 (2008).
Kalaitzakis, M. E., Graeber, M. B., Gentleman, S. M. & Pearce, R. K. Controversies over the staging of α-synuclein pathology in Parkinson's disease. Acta Neuropathol. 116, 125–128 (2008).
Braak, H. & Del Tredici, K. Invited Article: Nervous system pathology in sporadic Parkinson disease. Neurology 70, 1916–1925 (2008).
Jellinger, K. A. A critical reappraisal of current staging of Lewy-related pathology in human brain. Acta Neuropathol. 116, 1–16 (2008).
Li, J. Y. et al. Long-term surviving transplanted dopamine neurons exhibit α-synuclein accumulation and Lewy bodies. Mov. Disord. Soc. 12th Int. Congress Parkinson's Dis. Mov. Disord. LB13, 11–12 (2008).
Lobsiger, C. S. & Cleveland, D. W. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nature Neurosci. 10, 1355–1360 (2007).
McGeer, P. L., Itagaki, S., Boyes, B. E. & McGeer, E. G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology 38, 1285–1291 (1988).
Whitton, P. S. Inflammation as a causative factor in the aetiology of Parkinson's disease. Br. J. Pharmacol. 150, 963–976 (2007).
Duan, W. M., Widner, H. & Brundin, P. Temporal pattern of host responses against intrastriatal grafts of syngeneic, allogeneic or xenogeneic embryonic neuronal tissue in rats. Exp. Brain Res. 104, 227–242 (1995).
Griffin, W. S., Liu, L., Li, Y., Mrak, R. E. & Barger, S. W. Interleukin-1 mediates Alzheimer and Lewy body pathologies. J. Neuroinflammation 3, 5 (2006).
Shavali, S., Combs, C. K. & Ebadi, M. Reactive macrophages increase oxidative stress and alpha-synuclein nitration during death of dopaminergic neuronal cells in co-culture: relevance to Parkinson's disease. Neurochem. Res. 31, 85–94 (2006).
Jenner, P. Oxidative stress in Parkinson's disease. Ann. Neurol. 53 (Suppl. 3), S26–S36; discussion S36–S38 (2003).
Lotharius, J. & Brundin, P. Pathogenesis of Parkinson's disease: dopamine, vesicles and α-synuclein. Nature Rev. Neurosci. 3, 932–942 (2002).
Takahashi, M. et al. Oxidative stress-induced phosphorylation, degradation and aggregation of α-synuclein are linked to upregulated CK2 and cathepsin D. Eur. J. Neurosci. 26, 863–874 (2007).
Vila, M. et al. α-synuclein up-regulation in substantia nigra dopaminergic neurons following administration of the parkinsonian toxin MPTP. J. Neurochem. 74, 721–729 (2000).
Gopinath, G., Shetty, A. K. & Tandon, P. N. Ageing changes in the transplants of fetal substantia nigra grafted to striatum of adult rat. Neuroscience 40, 429–443 (1991).
Shtilerman, M. D., Ding, T. T. & Lansbury, P. T. Jr. Molecular crowding accelerates fibrillization of α-synuclein: could an increase in the cytoplasmic protein concentration induce Parkinson's disease? Biochemistry 41, 3855–3860 (2002).
Beal, M. F. Excitotoxicity and nitric oxide in Parkinson's disease pathogenesis. Ann. Neurol. 44, S110–S114 (1998).
Sonsalla, P. K., Albers, D. S. & Zeevalk, G. D. Role of glutamate in neurodegeneration of dopamine neurons in several animal models of parkinsonism. Amino Acids 14, 69–74 (1998).
Rutherford, A., Garcia-Munoz, M., Dunnett, S. B. & Arbuthnott, G. W. Electrophysiological demonstration of host cortical inputs to striatal grafts. Neurosci. Lett. 83, 275–281 (1987).
Doucet, G. et al. Host afferents into intrastriatal transplants of fetal ventral mesencephalon. Exp. Neurol. 106, 1–19 (1989).
Fisher, L. J., Young, S. J., Tepper, J. M., Groves, P. M. & Gage, F. H. Electrophysiological characteristics of cells within mesencephalon suspension grafts. Neuroscience 40, 109–122 (1991).
Siegel, G. J. & Chauhan, N. B. Neurotrophic factors in Alzheimer's and Parkinson's disease brain. Brain Res. Rev. 33, 199–227 (2000).
Smith, M. P. & Cass, W. A. GDNF reduces oxidative stress in a 6-hydroxydopamine model of Parkinson's disease. Neurosci. Lett. 412, 259–263 (2007).
Meyer-Luehmann, M. et al. Exogenous induction of cerebral β-amyloidogenesis is governed by agent and host. Science 313, 1781–1784 (2006).
Braak, H., Rub, U., Gai, W. P. & Del Tredici, K. Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural Transm. 110, 517–536 (2003).
Hardy, J. Expression of normal sequence pathogenic proteins for neurodegenerative disease contributes to disease risk: 'permissive templating' as a general mechanism underlying neurodegeneration. Biochem. Soc. Trans. 33, 578–581 (2005).
Mori, F. et al. α-synuclein pathology in the neostriatum in Parkinson's disease. Acta Neuropathol. 115, 453–459 (2008).
Kordower, J. H. & Sortwell, C. E. Neuropathology of fetal nigra transplants for Parkinson's disease. Prog. Brain Res. 127, 333–344 (2000).
Lee, H. J., Patel, S. & Lee, S. J. Intravesicular localization and exocytosis of α-synuclein and its aggregates. J. Neurosci. 25, 6016–6024 (2005).
Mollenhauer, B. et al. Direct quantification of CSF α-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp. Neurol. 14 Jun 2008 (doi:10.1016/j.expneurol.2008.06.004).
Borghi, R. et al. Full length α-synuclein is present in cerebrospinal fluid from Parkinson's disease and normal subjects. Neurosci. Lett. 287, 65–67 (2000).
El-Agnaf, O. M. et al. α-synuclein implicated in Parkinson's disease is present in extracellular biological fluids, including human plasma. FASEB J. 17, 1945–1947 (2003).
Tokuda, T. et al. Decreased α-synuclein in cerebrospinal fluid of aged individuals and subjects with Parkinson's disease. Biochem. Biophys. Res. Commun. 349, 162–166 (2006).
Lee, H. J. et al. Assembly-dependent endocytosis and clearance of extracellular α-synuclein. Int. J. Biochem. Cell Biol. 40, 1835–1849 (2008).
Ahn, K. J., Paik, S. R., Chung, K. C. & Kim, J. Amino acid sequence motifs and mechanistic features of the membrane translocation of α-synuclein. J. Neurochem. 97, 265–279 (2006).
Sung, J. Y. et al. Induction of neuronal cell death by Rab5A-dependent endocytosis of α-synuclein. J. Biol. Chem. 276, 27441–27448 (2001).
Hagell, P. et al. Sequential bilateral transplantation in Parkinson's disease: effects of the second graft. Brain 122, 1121–1132 (1999).
Acknowledgements
This work was supported by grants from the Swedish Research Council, Swedish Parkinson Foundation, the Nordic Center of Excellence on Neurodegeneration and The Strong Research Environment of the Swedish Research Council (NeuroFortis). The Queen Square Brain Bank is supported by the Reta Lila Weston Institute of Neurological Studies, the Progressive Supranuclear Palsy (Europe) Association and BrainNet Europe. T.R. and J.L.H. are supported by grants from the Alzheimer's Research Trust and the Sarah Matheson Trust.
Author information
Authors and Affiliations
Related links
Rights and permissions
About this article
Cite this article
Brundin, P., Li, JY., Holton, J. et al. Research in motion: the enigma of Parkinson's disease pathology spread. Nat Rev Neurosci 9, 741–745 (2008). https://doi.org/10.1038/nrn2477
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn2477