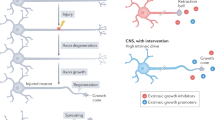
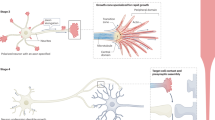
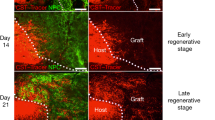
Key Points
-
Adult mammalian CNS regeneration is limited by a combination of intrinsic and extrinsic inhibitory barriers. This differs from the extraordinary ability to form short- and long-distance connections and complex circuits during nervous system development.
-
Various signalling molecules guide developing neuronal branches. Many of these molecules persist in adults, but in different quantitative and qualitative distributions.
-
The immature nervous system is refined by experience-dependent plasticity, resulting in the pruning of unnecessary connections and strengthening of useful ones. Mechanisms responsible for consolidating these refinements largely prevent further plastic changes, and secondarily inhibit regenerative responses in the context of injury.
-
Local network circuits termed central pattern generators (CPGs) regulate semi-automatic behaviours such as ambulation. CPG plasticity and adaptation depend on sensory feedback and voluntary input.
-
Intrinsic barriers to CNS regeneration include an unfavourable intracellular second messenger milieu as well as the inability to use regeneration-associated genes.
-
Extrinsic barriers to CNS regeneration include inhibitory molecules produced by oligodendrocytes, astrocytes and inflammatory cells. The altered distribution of growth and guidance factors in the adult relative to the developing nervous systems represents another extrinsic barrier to effective regeneration.
-
Advances using stem cells, neurotrophins and antagonists of extracellular inhibitors have resulted in a limited degree of CNS regeneration so far. Better approaches are required to recapitulate the precision of developmental growth, guidance and plasticity mechanisms.
-
One strategy to mimic the developmental milieu requires better understanding of the changes in distribution of key guidance molecules during and after development.
-
Rehabilitation approaches that maximize sensory feedback to CPGs will optimize the adaptation to loss of descending voluntary input.
Abstract
The precise wiring of the adult mammalian CNS originates during a period of stunning growth, guidance and plasticity that occurs during and shortly after development. When injured in adults, this intricate system fails to regenerate. Even when the obstacles to regeneration are cleared, growing adult CNS fibres usually remain misdirected and fail to reform functional connections. Here, we attempt to fill an important niche related to the topics of nervous system development and regeneration. We specifically contrast the difficulties faced by growing fibres within the adult context to the precise circuit-forming capabilities of developing fibres. In addition to focusing on methods to stimulate growth in the adult, we also expand on approaches to recapitulate development itself.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hemmati-Brivanlou, A., Kelly, O. G. & Melton, D. A. Follistatin, an antagonist of activin, is expressed in the Spemann organizer and displays direct neuralizing activity. Cell 77, 283–295 (1994).
Lamb, T. M. et al. Neural induction by the secreted polypeptide noggin. Science 262, 713–718 (1993).
Lamb, T. M. & Harland, R. M. Fibroblast growth factor is a direct neural inducer, which combined with noggin generates anterior-posterior neural pattern. Development 121, 3627–3636 (1995).
Durston, A. J. et al. Retinoic acid causes an anteroposterior transformation in the developing central nervous system. Nature 340, 140–144 (1989).
Tanabe, Y. & Jessell, T. M. Diversity and pattern in the developing spinal cord. Science 274, 1115–1123 (1996).
Muroyama, Y., Fujihara, M., Ikeya, M., Kondoh, H. & Takada, S. Wnt signaling plays an essential role in neuronal specification of the dorsal spinal cord. Genes Dev. 16, 548–553 (2002).
Yamada, T., Pfaff, S. L., Edlund, T. & Jessell, T. M. Control of cell pattern in the neural tube: motor neuron induction by diffusible factors from notochord and floor plate. Cell 73, 673–686 (1993).
Echelard, Y. et al. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 75, 1417–1430 (1993).
Charron, F. & Tessier-Lavigne, M. Novel brain wiring functions for classical morphogens: a role as graded positional cues in axon guidance. Development 132, 2251–2262 (2005).
Liu, Y. et al. Ryk-mediated Wnt repulsion regulates posterior-directed growth of corticospinal tract. Nature Neurosci. 8, 1151–1159 (2005). A convincing demonstration of gradient-guided CST development and the increasingly recognized multipurpose role of morphogens.
Charron, F., Stein, E., Jeong, J., McMahon, A. P. & Tessier-Lavigne, M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell 113, 11–23 (2003).
Bourikas, D. et al. Sonic hedgehog guides commissural axons along the longitudinal axis of the spinal cord. Nature Neurosci. 8, 297–304 (2005).
Lie, D. C. et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature 437, 1370–1375 (2005).
Lai, K., Kaspar, B. K., Gage, F. H. & Schaffer, D. V. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nature Neurosci. 6, 21–27 (2003).
Chen, J., Magavi, S. S. & Macklis, J. D. Neurogenesis of corticospinal motor neurons extending spinal projections in adult mice. Proc. Natl Acad. Sci. USA 101, 16357–16362 (2004).
Doetsch, F., Caille, I., Lim, D. A., Garcia-Verdugo, J. M. & Alvarez-Buylla, A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97, 703–716 (1999).
Horner, P. J. et al. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J. Neurosci. 20, 2218–2228 (2000).
Kempermann, G. & Gage, F. H. Neurogenesis in the adult hippocampus. Novartis Found. Symp. 231, 220–235; discussion 235–241, 302–306 (2000).
Leavitt, B. R., Hernit-Grant, C. S. & Macklis, J. D. Mature astrocytes transform into transitional radial glia within adult mouse neocortex that supports directed migration of transplanted immature neurons. Exp. Neurol. 157, 43–57 (1999).
Sotelo, C., Alvarado-Mallart, R. M., Frain, M. & Vernet, M. Molecular plasticity of adult Bergmann fibers is associated with radial migration of grafted Purkinje cells. J. Neurosci. 14, 124–133 (1994). Together with reference 19, this suggests that adult differentiated CNS glia retain the ability to revert to the radial glia phenotype to guide endogenous or exogenous immature migrating neurons.
Dickson, B. J. Molecular mechanisms of axon guidance. Science 298, 1959–1964 (2002).
Koeberle, P. D. & Bahr, M. Growth and guidance cues for regenerating axons: where have they gone? J. Neurobiol. 59, 162–180 (2004).
Serafini, T. et al. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell 78, 409–424 (1994).
Kennedy, T. E., Serafini, T., de la Torre, J. R. & Tessier-Lavigne, M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell 78, 425–435 (1994).
Harris, R., Sabatelli, L. M. & Seeger, M. A. Guidance cues at the Drosophila CNS midline: identification and characterization of two Drosophila Netrin/UNC-6 homologs. Neuron 17, 217–228 (1996).
Brose, K. et al. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell 96, 795–806 (1999).
Kidd, T., Bland, K. S. & Goodman, C. S. Slit is the midline repellent for the robo receptor in Drosophila. Cell 96, 785–94 (1999).
Li, H. S. et al. Vertebrate slit, a secreted ligand for the transmembrane protein roundabout, is a repellent for olfactory bulb axons. Cell 96, 807–818 (1999).
Wang, K. H. et al. Biochemical purification of a mammalian slit protein as a positive regulator of sensory axon elongation and branching. Cell 96, 771–784 (1999).
Sabatier, C. et al. The divergent Robo family protein rig-1/Robo3 is a negative regulator of slit responsiveness required for midline crossing by commissural axons. Cell 117, 157–169 (2004). Shows that, unlike isoforms 1 and 2, ROBO3 facilitates midline attraction rather than repulsion. In fact, ROBO3 is required for midline axon attraction, as its mutation in humans leads to the disorder HGPPS, in which multiple CNS tracts fail to cross the midline (see reference 60).
Stein, E. & Tessier-Lavigne, M. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science 291, 1928–1938 (2001).
Tessier-Lavigne, M. & Goodman, C. S. The molecular biology of axon guidance. Science 274, 1123–1133 (1996).
Klein, R. Eph/ephrin signaling in morphogenesis, neural development and plasticity. Curr. Opin. Cell Biol. 16, 580–589 (2004).
Martinez, A. & Soriano, E. Functions of ephrin/Eph interactions in the development of the nervous system: emphasis on the hippocampal system. Brain Res. Brain Res. Rev. 49, 211–226 (2005).
Brown, A. et al. Topographic mapping from the retina to the midbrain is controlled by relative but not absolute levels of EphA receptor signaling. Cell 102, 77–88 (2000).
Feldheim, D. A. et al. Genetic analysis of ephrin-A2 and ephrin-A5 shows their requirement in multiple aspects of retinocollicular mapping. Neuron 25, 563–574 (2000).
Monnier, P. P. et al. RGM is a repulsive guidance molecule for retinal axons. Nature 419, 392–395 (2002).
Rajagopalan, S. et al. Neogenin mediates the action of repulsive guidance molecule. Nature Cell Biol. 6, 756–762 (2004).
Benson, M. D. et al. Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc. Natl Acad. Sci. USA 102, 10694–10699 (2005).
Hata, K. et al. RGMa inhibition promotes axonal growth and recovery after spinal cord injury. J. Cell Biol. 173, 47–58 (2006).
Bach, H., Feldheim, D. A., Flanagan, J. G. & Scalia, F. Persistence of graded EphA/Ephrin-A expression in the adult frog visual system. J. Comp. Neurol. 467, 549–565 (2003).
Liu, B. P. & Strittmatter, S. M. Semaphorin-mediated axonal guidance via Rho-related G proteins. Curr. Opin. Cell Biol. 13, 619–626 (2001).
Kolodkin, A. L., Matthes, D. J. & Goodman, C. S. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell 75, 1389–1399 (1993).
Pasterkamp, R. J., Peschon, J. J., Spriggs, M. K. & Kolodkin, A. L. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature 424, 398–405 (2003).
Leonardo, E. D. et al. Vertebrate homologues of C. elegans UNC-5 are candidate netrin receptors. Nature 386, 833–838 (1997).
Shewan, D., Dwivedy, A., Anderson, R. & Holt, C. E. Age-related changes underlie switch in netrin-1 responsiveness as growth cones advance along visual pathway. Nature Neurosci. 5, 955–962 (2002).
Song, H. et al. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science 281, 1515–1518 (1998).
Song, H. J. & Poo, M. M. Signal transduction underlying growth cone guidance by diffusible factors. Curr. Opin. Neurobiol. 9, 355–363 (1999).
Chen, D. F., Jhaveri, S. & Schneider, G. E. Intrinsic changes in developing retinal neurons result in regenerative failure of their axons. Proc. Natl Acad. Sci. USA 92, 7287–7291 (1995).
Fawcett, J. W. Astrocytic and neuronal factors affecting axon regeneration in the damaged central nervous system. Cell Tissue Res. 290, 371–377 (1997).
Cai, D. et al. Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J. Neurosci. 21, 4731–4739 (2001).
Dottori, M. et al. EphA4 (Sek1) receptor tyrosine kinase is required for the development of the corticospinal tract. Proc. Natl Acad. Sci. USA 95, 13248–13253 (1998). One of the first publications to link CST development to specific guidance molecules. Also describes the 'kangaroo-like' gait displayed by mice with various mutations affecting CST development.
Finger, J. H. et al. The netrin 1 receptors Unc5h3 and Dcc are necessary at multiple choice points for the guidance of corticospinal tract axons. J. Neurosci. 22, 10346–10356 (2002).
Kullander, K. et al. Ephrin-B3 is the midline barrier that prevents corticospinal tract axons from recrossing, allowing for unilateral motor control. Genes Dev. 15, 877–888 (2001).
Polleux, F., Morrow, T. & Ghosh, A. Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature 404, 567–573 (2000).
Kullander, K. et al. Role of EphA4 and EphrinB3 in local neuronal circuits that control walking. Science 299, 1889–1892 (2003). Previous papers (references 52 and 54) hinted that EphA4 or ephrin B3 knockout lead to a 'kangaroo-like' gait due to inappropriate CST midline crossing. Surprisingly, this paper demonstrates that the phenotype of these mice actually stems from aberrant crossing of segmental local interneurons within the CPG, rather than from defective CST development.
Cohen, N. R. et al. Errors in corticospinal axon guidance in mice lacking the neural cell adhesion molecule L1. Curr. Biol. 8, 26–33 (1998).
Graf, W. D. et al. Diffusion-weighted magnetic resonance imaging in boys with neural cell adhesion molecule L1 mutations and congenital hydrocephalus. Ann. Neurol. 47, 113–117 (2000).
Castellani, V., Chedotal, A., Schachner, M., Faivre-Sarrailh, C. & Rougon, G. Analysis of the L1-deficient mouse phenotype reveals cross-talk between Sema3A and L1 signaling pathways in axonal guidance. Neuron 27, 237–249 (2000).
Jen, J. C. et al. Mutations in a human ROBO gene disrupt hindbrain axon pathway crossing and morphogenesis. Science 304, 1509–1513 (2004). Demonstrates in a human disease population the requirement for ROBO3 to mediate midline attraction and crossing in multiple CNS tracts (see also reference 30).
Oppenheim, R. W. Cell death during development of the nervous system. Annu. Rev. Neurosci. 14, 453–501 (1991).
Luo, L. & O'Leary, D. D. Axon retraction and degeneration in development and disease. Annu. Rev. Neurosci. 28, 127–156 (2005).
Levi-Montalcini, R. & Levi, G. Les conséquences de la destruction d'un territoire d'innervation périphérique sur le développement des centres nerveux correspondants dans l'embryon de poulet. Arch. Biol. 53, 537–545 (1942).
Hamburger, V. The effects of wing bug extirpation in chick embryos on the development of the central nervous system. J. Exp. Zool. 68, 449–494 (1934).
Hamburger, V. Cell death in the development of the lateral motor column of the chick embryo. J. Comp. Neurol. 160, 535–546 (1975).
Hollyday, M. & Hamburger, V. Reduction of the naturally occurring motor neuron loss by enlargement of the periphery. J. Comp. Neurol. 170, 311–320 (1976).
Goldberg, J. L. How does an axon grow? Genes Dev. 17, 941–958 (2003).
Mendell, L. M. & Arvanian, V. L. Diversity of neurotrophin action in the postnatal spinal cord. Brain Res. Brain Res. Rev. 40, 230–239 (2002).
Snider, W. D., Elliott, J. L. & Yan, Q. Axotomy-induced neuronal death during development. J. Neurobiol. 23, 1231–1246 (1992).
Anand, U. et al. The effect of neurotrophic factors on morphology, TRPV1 expression and capsaicin responses of cultured human DRG sensory neurons. Neurosci. Lett. 399, 51–56 (2006).
Hensch, T. K. Critical period plasticity in local cortical circuits. Nature Rev. Neurosci. 6, 877–888 (2005).
Knudsen, E. I. Instructed learning in the auditory localization pathway of the barn owl. Nature 417, 322–328 (2002).
Knudsen, E. I. Capacity for plasticity in the adult owl auditory system expanded by juvenile experience. Science 279, 1531–1533 (1998).
Hubel, D. H., Wiesel, T. N. & LeVay, S. Functional architecture of area 17 in normal and monocularly deprived macaque monkeys. Cold Spring Harb. Symp. Quant. Biol. 40, 581–589 (1976).
Shatz, C. J. & Stryker, M. P. Ocular dominance in layer IV of the cat's visual cortex and the effects of monocular deprivation. J. Physiol. 281, 267–283 (1978).
Antonini, A., Fagiolini, M. & Stryker, M. P. Anatomical correlates of functional plasticity in mouse visual cortex. J. Neurosci. 19, 4388–4406 (1999).
Hensch, T. K. et al. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science 282, 1504–1508 (1998).
Fagiolini, M. & Hensch, T. K. Inhibitory threshold for critical-period activation in primary visual cortex. Nature 404, 183–186 (2000).
Huang, Z. J. et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 98, 739–755 (1999).
Fagiolini, M. et al. Specific GABAA circuits for visual cortical plasticity. Science 303, 1681–1683 (2004).
Keller-Peck, C. R. et al. Asynchronous synapse elimination in neonatal motor units: studies using GFP transgenic mice. Neuron 31, 381–394 (2001).
De Paola, V. et al. Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron 49, 861–875 (2006).
Holtmaat, A. J. et al. Transient and persistent dendritic spines in the neocortex in vivo. Neuron 45, 279–291 (2005).
Lee, W. C. et al. Dynamic remodeling of dendritic arbors in GABAergic interneurons of adult visual cortex. PLoS Biol 4, e29 (2006).
Duysens, J. & Van de Crommert, H. W. Neural control of locomotion; the central pattern generator from cats to humans. Gait Posture 7, 131–141 (1998).
Dietz, V. Spinal cord pattern generators for locomotion. Clin. Neurophysiol. 114, 1379–1389 (2003).
Edgerton, V. R., Tillakaratne, N. J., Bigbee, A. J., de Leon, R. D. & Roy, R. R. Plasticity of the spinal neural circuitry after injury. Annu. Rev. Neurosci. 27, 145–167 (2004).
Suster, M. L. & Bate, M. Embryonic assembly of a central pattern generator without sensory input. Nature 416, 174–178 (2002).
Pearson, K. G. Generating the walking gait: role of sensory feedback. Prog. Brain Res. 143, 123–129 (2004).
Walton, K. D., Lieberman, D., Llinas, A., Begin, M. & Llinas, R. R. Identification of a critical period for motor development in neonatal rats. Neuroscience 51, 763–767 (1992).
Durkovic, R. G. & Damianopoulos, E. N. Forward and backward classical conditioning of the flexion reflex in the spinal cat. J. Neurosci. 6, 2921–2925 (1986).
Edgerton, V. R. et al. Potential of adult mammalian lumbosacral spinal cord to execute and acquire improved locomotion in the absence of supraspinal input. J. Neurotrauma 9, S119–S128 (1992).
de Leon, R. D., Hodgson, J. A., Roy, R. R. & Edgerton, V. R. Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J. Neurophysiol. 79, 1329–1340 (1998). One of many publications from the Edgerton group emphasizing the improved plasticity of intrinsic spinal cord circuits that is achieved with sensorimotor feedback training.
Kudo, N., Nishimaru, H. & Nakayama, K. Developmental changes in rhythmic spinal neuronal activity in the rat fetus. Prog. Brain Res. 143, 49–55 (2004).
Allain, A. E., Meyrand, P. & Branchereau, P. Ontogenic changes of the spinal GABAergic cell population are controlled by the serotonin (5-HT) system: implication of 5-HT1 receptor family. J. Neurosci. 25, 8714–8724 (2005).
McGee, A. W., Yang, Y., Fischer, Q. S., Daw, N. W. & Strittmatter, S. M. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science 309, 2222–2226 (2005).
Pizzorusso, T. et al. Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298, 1248–1251 (2002). Using both immunohistological and functional techniques, together with reference 96, the authors begin to elucidate the molecular mechanisms of ocular dominance plasticity, identifying the key roles of CSPGs and MAIs.
Lander, C., Kind, P., Maleski, M. & Hockfield, S. A family of activity-dependent neuronal cell-surface chondroitin sulfate proteoglycans in cat visual cortex. J. Neurosci. 17, 1928–1939 (1997).
Ferretti, P., Zhang, F. & O'Neill, P. Changes in spinal cord regenerative ability through phylogenesis and development: lessons to be learnt. Dev. Dyn. 226, 245–256 (2003). An interesting review that goes into more depth on the cellular and molecular mechanisms underlying the starkly contrasting ability of lower versus higher vertebrates to regenerate the injured CNS.
Benowitz, L. I., Leon, S., Tabibiazar, R., Jing, Y. & Irwin, N. in Axonal Regeneration in the Central Nervous System (eds Ingoglia, N. A. & Murray, M.) 45–66 (Marcel Dekker, New York, 2001).
Saunders, N. R. et al. Development of walking, swimming and neuronal connections after complete spinal cord transection in the neonatal opossum, Monodelphis domestica. J. Neurosci. 18, 339–355 (1998).
Tom, V. J., Steinmetz, M. P., Miller, J. H., Doller, C. M. & Silver, J. Studies on the development and behavior of the dystrophic growth cone, the hallmark of regeneration failure, in an in vitro model of the glial scar and after spinal cord injury. J. Neurosci. 24, 6531–6539 (2004).
Kerschensteiner, M., Schwab, M. E., Lichtman, J. W. & Misgeld, T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nature Med. 11, 572–577 (2005). A beautiful application of in vivo two-photon imaging of the mammalian CNS response to axonal injury. Confirms some of Ramón y Cajal's ingenious insights into transected fibre degeneration and regeneration.
Ramón y Cajal, S., DeFelipe, J. & Jones, E. G. Cajal's Degeneration and Regeneration of the Nervous System (Oxford Univ. Press, New York, 1991).
Fawcett, J. W., Housden, E., Smith-Thomas, L. & Meyer, R. L. The growth of axons in three-dimensional astrocyte cultures. Dev. Biol. 135, 449–458 (1989).
Goldberg, J. L., Klassen, M. P., Hua, Y. & Barres, B. A. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science 296, 1860–1864 (2002).
Qiu, J. et al. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron 34, 895–903 (2002).
Gao, Y. et al. Activated CREB is sufficient to overcome inhibitors in myelin and promote spinal axon regeneration in vivo. Neuron 44, 609–621 (2004).
Neumann, S. & Woolf, C. J. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron 23, 83–91 (1999).
Bonilla, I. E., Tanabe, K. & Strittmatter, S. M. Small proline-rich repeat protein 1A is expressed by axotomized neurons and promotes axonal outgrowth. J. Neurosci. 22, 1303–1315 (2002).
Cai, D. et al. Arginase I and polyamines act downstream from cyclic AMP in overcoming inhibition of axonal growth MAG and myelin in vitro. Neuron 35, 711–719 (2002).
Marklund, N. et al. Selective temporal and regional alterations of Nogo-A and small proline-rich repeat protein 1A (SPRR1A) but not Nogo-66 receptor (NgR) occur following traumatic brain injury in the rat. Exp. Neurol. 197, 70–83 (2006).
Schmitt, A. B. et al. GAP-43 (B-50) and C-Jun are up-regulated in axotomized neurons of Clarke's nucleus after spinal cord injury in the adult rat. Neurobiol. Dis. 6, 122–130 (1999).
Skene, J. H. & Willard, M. Characteristics of growth-associated polypeptides in regenerating toad retinal ganglion cell axons. J. Neurosci. 1, 419–426 (1981).
Tanabe, K., Bonilla, I., Winkles, J. A. & Strittmatter, S. M. Fibroblast growth factor-inducible-14 is induced in axotomized neurons and promotes neurite outgrowth. J. Neurosci. 23, 9675–9686 (2003).
Raivich, G. et al. The AP-1 transcription factor c-Jun is required for efficient axonal regeneration. Neuron 43, 57–67 (2004).
Fournier, A. E., GrandPre, T. & Strittmatter, S. M. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature 409, 341–346 (2001).
Mingorance, A. et al. Regulation of Nogo and Nogo receptor during the development of the entorhino-hippocampal pathway and after adult hippocampal lesions. Mol. Cell. Neurosci. 26, 34–49 (2004).
Manitt, C., Thompson, K. M. & Kennedy, T. E. Developmental shift in expression of netrin receptors in the rat spinal cord: predominance of UNC-5 homologues in adulthood. J. Neurosci. Res. 77, 690–700 (2004).
Ellezam, B., Selles-Navarro, I., Manitt, C., Kennedy, T. E. & McKerracher, L. Expression of netrin-1 and its receptors DCC and UNC-5H2 after axotomy and during regeneration of adult rat retinal ganglion cells. Exp. Neurol. 168, 105–115 (2001).
David, S. & Aguayo, A. J. Axonal elongation into peripheral nervous system 'bridges' after central nervous system injury in adult rats. Science 214, 931–933 (1981). One of a series of papers from Aguayo's group that revives work that was first performed by Tello and Ramón y Cajal, demonstrating that CNS axons can indeed regenerate if provided with a permissive environment.
Schwab, M. E. & Thoenen, H. Dissociated neurons regenerate into sciatic but not optic nerve explants in culture irrespective of neurotrophic factors. J. Neurosci. 5, 2415–2423 (1985).
Berry, M. Post-injury myelin-breakdown products inhibit axonal growth: an hypothesis to explain the failure of axonal regeneration in the mammalian central nervous system. Bibl. Anat. 23, 1–11 (1982). Demonstrates that the stimulatory effect of peripheral nerve grafts on CNS regeneration does not depend on neurotrophic factors unique to the peripheral milieu, but more likely reflects the presence of inhibitory factors in the CNS environment.
Schwab, M. E. & Bartholdi, D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol. Rev. 76, 319–370 (1996).
Domeniconi, M. et al. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron 35, 283–290 (2002).
Liu, B. P., Fournier, A., GrandPre, T. & Strittmatter, S. M. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science 297, 1190–1193 (2002).
Wang, K. C. et al. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature 417, 941–944 (2002).
Huber, A. B., Weinmann, O., Brosamle, C., Oertle, T. & Schwab, M. E. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J. Neurosci. 22, 3553–3567 (2002).
Wang, X. et al. Localization of Nogo-A and Nogo-66 receptor proteins at sites of axon–myelin and synaptic contact. J. Neurosci. 22, 5505–5515 (2002).
Oertle, T. & Schwab, M. E. Nogo and its paRTNers. Trends Cell Biol. 13, 187–194 (2003).
Yiu, G. & He, Z. Glial inhibition of CNS axon regeneration. Nature Rev. Neurosci. 7, 617–627 (2006).
Schwab, M. E. Repairing the injured spinal cord. Science 295, 1029–1031 (2002).
Thuret, S., Moon, L. D. F. & Gage, F. H. Therapeutic interventions after spinal cord injury. Nature Rev. Neurosci. 7, 628–643 (2006).
Fricker-Gates, R. A., Shin, J. J., Tai, C. C., Catapano, L. A. & Macklis, J. D. Late-stage immature neocortical neurons reconstruct interhemispheric connections and form synaptic contacts with increased efficiency in adult mouse cortex undergoing targeted neurodegeneration. J. Neurosci. 22, 4045–4056 (2002).
Lepore, A. C. et al. Long-term fate of neural precursor cells following transplantation into developing and adult CNS. Neuroscience 139, 513–530 (2006).
Bregman, B. S. et al. Transplants and neurotrophic factors increase regeneration and recovery of function after spinal cord injury. Prog. Brain Res. 137, 257–273 (2002).
Cummings, B. J. et al. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc. Natl Acad. Sci. USA 102, 14069–14074 (2005).
Sheen, V. L. & Macklis, J. D. Targeted neocortical cell death in adult mice guides migration and differentiation of transplanted embryonic neurons. J. Neurosci. 15, 8378–8392 (1995).
Bregman, B. S., McAtee, M., Dai, H. N. & Kuhn, P. L. Neurotrophic factors increase axonal growth after spinal cord injury and transplantation in the adult rat. Exp. Neurol. 148, 475–494 (1997).
Rao, M. S., Hattiangady, B. & Shetty, A. K. Fetal hippocampal CA3 cell grafts enriched with FGF-2 and BDNF exhibit robust long-term survival and integration and suppress aberrant mossy fiber sprouting in the injured middle-aged hippocampus. Neurobiol. Dis. 21, 276–290 (2006).
Dumesnil-Bousez, N. & Sotelo, C. Partial reconstruction of the adult Lurcher cerebellar circuitry by neural grafting. Neuroscience 55, 1–21 (1993).
Nakatomi, H. et al. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 110, 429–441 (2002).
Magavi, S. S., Leavitt, B. R. & Macklis, J. D. Induction of neurogenesis in the neocortex of adult mice. Nature 405, 951–955 (2000).
Arvidsson, A., Collin, T., Kirik, D., Kokaia, Z. & Lindvall, O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nature Med. 8, 963–970 (2002).
Scharff, C., Kirn, J. R., Grossman, M., Macklis, J. D. & Nottebohm, F. Targeted neuronal death affects neuronal replacement and vocal behavior in adult songbirds. Neuron 25, 481–492 (2000).
Kempermann, G., van Praag, H. & Gage, F. H. Activity-dependent regulation of neuronal plasticity and self repair. Prog. Brain Res. 127, 35–48 (2000).
Lu, P., Yang, H., Jones, L. L., Filbin, M. T. & Tuszynski, M. H. Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. J. Neurosci. 24, 6402–6409 (2004).
Harper, J. M. et al. Axonal growth of embryonic stem cell-derived motoneurons in vitro and in motoneuron-injured adult rats. Proc. Natl Acad. Sci. USA 101, 7123–7128 (2004).
Bomze, H. M., Bulsara, K. R., Iskandar, B. J., Caroni, P. & Skene, J. H. Spinal axon regeneration evoked by replacing two growth cone proteins in adult neurons. Nature Neurosci. 4, 38–43 (2001).
Grill, R., Murai, K., Blesch, A., Gage, F. H. & Tuszynski, M. H. Cellular delivery of neurotrophin-3 promotes corticospinal axonal growth and partial functional recovery after spinal cord injury. J. Neurosci. 17, 5560–5572 (1997).
Jakeman, L. B., Wei, P., Guan, Z. & Stokes, B. T. Brain-derived neurotrophic factor stimulates hindlimb stepping and sprouting of cholinergic fibers after spinal cord injury. Exp. Neurol. 154, 170–184 (1998).
Houweling, D. A. et al. Local application of collagen containing brain-derived neurotrophic factor decreases the loss of function after spinal cord injury in the adult rat. Neurosci. Lett. 251, 193–196 (1998).
Novikova, L., Novikov, L. & Kellerth, J. O. Brain-derived neurotrophic factor reduces necrotic zone and supports neuronal survival after spinal cord hemisection in adult rats. Neurosci. Lett. 220, 203–206 (1996).
Jean, I., Lavialle, C., Barthelaix-Pouplard, A. & Fressinaud, C. Neurotrophin-3 specifically increases mature oligodendrocyte population and enhances remyelination after chemical demyelination of adult rat CNS. Brain Res. 972, 110–118 (2003).
Hendriks, W. T., Ruitenberg, M. J., Blits, B., Boer, G. J. & Verhaagen, J. Viral vector-mediated gene transfer of neurotrophins to promote regeneration of the injured spinal cord. Prog. Brain Res. 146, 451–476 (2004).
Palmer, T. D., Markakis, E. A., Willhoite, A. R., Safar, F. & Gage, F. H. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J. Neurosci. 19, 8487–8497 (1999).
Kim, J. E., Liu, B. P., Park, J. H. & Strittmatter, S. M. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron 44, 439–451 (2004).
Zheng, B. et al. Genetic deletion of the Nogo receptor does not reduce neurite inhibition in vitro or promote corticospinal tract regeneration in vivo. Proc. Natl Acad. Sci. USA 102, 1205–1210 (2005).
Lee, J. K., Kim, J. E., Sivula, M. & Strittmatter, S. M. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J. Neurosci. 24, 6209–6217 (2004).
Li, S. et al. Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J. Neurosci. 24, 10511–10520 (2004). Shows that blocking the receptor for three major MAIs enhances CST regeneration following SCI, but growth is limited and proceeds along ectopic pathways.
Markus, T. M. et al. Recovery and brain reorganization after stroke in adult and aged rats. Ann. Neurol. 58, 950–953 (2005).
Liebscher, T. et al. Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann. Neurol. 58, 706–719 (2005). Shows that the blockade of Nogo-A's unique amino-terminal domain also improves axon regeneration, again in a limited, non-fasciculated pattern.
Raineteau, O. & Schwab, M. E. Plasticity of motor systems after incomplete spinal cord injury. Nature Rev. Neurosci. 2, 263–273 (2001).
Cao, Q. et al. Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. J. Neurosci. 25, 6947–6957 (2005).
Niederost, B., Oertle, T., Fritsche, J., McKinney, R. A. & Bandtlow, C. E. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J. Neurosci. 22, 10368–10376 (2002).
Monnier, P. P., Sierra, A., Schwab, J. M., Henke-Fahle, S. & Mueller, B. K. The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol. Cell. Neurosci. 22, 319–330 (2003).
Dergham, P. et al. Rho signaling pathway targeted to promote spinal cord repair. J. Neurosci. 22, 6570–6577 (2002).
Ellezam, B. et al. Inactivation of intracellular Rho to stimulate axon growth and regeneration. Prog. Brain Res. 137, 371–380 (2002).
Bertrand, J., Winton, M. J., Rodriguez-Hernandez, N., Campenot, R. B. & McKerracher, L. Application of Rho antagonist to neuronal cell bodies promotes neurite growth in compartmented cultures and regeneration of retinal ganglion cell axons in the optic nerve of adult rats. J. Neurosci. 25, 1113–1121 (2005).
Fournier, A. E., Takizawa, B. T. & Strittmatter, S. M. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J. Neurosci. 23, 1416–1423 (2003).
Jain, A., Brady-Kalnay, S. M. & Bellamkonda, R. V. Modulation of Rho GTPase activity alleviates chondroitin sulfate proteoglycan-dependent inhibition of neurite extension. J. Neurosci. Res. 77, 299–307 (2004).
Cafferty, W. B. J. & Strittmatter, S. M. Nogo/NgR mediated plasticity after spinal cord injury. Soc. Neurosci. Abstr. 719.8 (2005).
Bradbury, E. J. et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416, 636–640 (2002).
Li, Y., Field, P. M. & Raisman, G. Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells. Science 277, 2000–2002 (1997).
Li, Y., Sauve, Y., Li, D., Lund, R. D. & Raisman, G. Transplanted olfactory ensheathing cells promote regeneration of cut adult rat optic nerve axons. J. Neurosci. 23, 7783–7788 (2003).
Mueller, B. K., Mack, H. & Teusch, N. Rho kinase, a promising drug target for neurological disorders. Nature Rev. Drug Discov. 4, 387–398 (2005).
Bareyre, F. M. et al. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nature Neurosci. 7, 269–277 (2004). A comprehensive demonstration of adult CNS plasticity in the context of incomplete SCI. An atypical but functional circuit bridges the lesion.
Barbeau, H. & Rossignol, S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 412, 84–95 (1987).
Rossignol, S. et al. Determinants of locomotor recovery after spinal injury in the cat. Prog. Brain Res. 143, 163–172 (2004). An extremely informative review from one of the giants in the field. Discusses the remarkable adaptability of the feline spinal cord locomotor CPG, and the roles of sensory feedback and neurotransmitters.
De Leon, R. D., Hodgson, J. A., Roy, R. R. & Edgerton, V. R. Full weight-bearing hindlimb standing following stand training in the adult spinal cat. J. Neurophysiol. 80, 83–91 (1998).
Bouyer, L. J. & Rossignol, S. The contribution of cutaneous inputs to locomotion in the intact and the spinal cat. Ann. NY Acad. Sci. 860, 508–512 (1998).
Fung, J. & Barbeau, H. Effects of conditioning cutaneomuscular stimulation on the soleus H-reflex in normal and spastic paretic subjects during walking and standing. J. Neurophysiol. 72, 2090–2104 (1994).
Bouyer, L. J. & Rossignol, S. Contribution of cutaneous inputs from the hindpaw to the control of locomotion. II. Spinal cats. J. Neurophysiol. 90, 3640–3653 (2003).
Dietz, V., Colombo, G., Jensen, L. & Baumgartner, L. Locomotor capacity of spinal cord in paraplegic patients. Ann. Neurol. 37, 574–582 (1995).
Wernig, A., Muller, S., Nanassy, A. & Cagol, E. Laufband therapy based on 'rules of spinal locomotion' is effective in spinal cord injured persons. Eur. J. Neurosci. 7, 823–829 (1995).
Dobkin, B. et al. Weight-supported treadmill vs over-ground training for walking after acute incomplete SCI. Neurology 66, 484–493 (2006).
Wolpaw, J. R. Treadmill training after spinal cord injury: good but not better. Neurology 66, 466–467 (2006). So far, references 186 and 187 report the most comprehensive and well-controlled clinical trial of BWSTT for SCI. The 'negative' result probably reflects the unexpectedly good outcome of the control group, which itself might represent improved rehabilitative therapy in general. This trial emphasizes the improving outlook for SCI patients with incomplete injuries.
Wirz, M., Colombo, G. & Dietz, V. Long term effects of locomotor training in spinal humans. J. Neurol. Neurosurg. Psychiatr. 71, 93–96 (2001).
Curt, A., Schwab, M. E. & Dietz, V. Providing the clinical basis for new interventional therapies: refined diagnosis and assessment of recovery after spinal cord injury. Spinal Cord 42, 1–6 (2004).
McGee, A. W. & Strittmatter, S. M. The Nogo-66 receptor: focusing myelin inhibition of axon regeneration. Trends Neurosci. 26, 193–198 (2003).
Grados-Munro, E. M. & Fournier, A. E. Myelin-associated inhibitors of axon regeneration. J. Neurosci. Res. 74, 479–485 (2003).
Schwab, M. E. Nogo and axon regeneration. Curr. Opin. Neurobiol. 14, 118–124 (2004).
McKerracher, L. et al. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron 13, 805–811 (1994).
Mukhopadhyay, G., Doherty, P., Walsh, F. S., Crocker, P. R. & Filbin, M. T. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron 13, 757–767 (1994).
Bartsch, U. et al. Lack of evidence that myelin-associated glycoprotein is a major inhibitor of axonal regeneration in the CNS. Neuron 15, 1375–1381 (1995).
Chen, M. S. et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 403, 434–439 (2000).
GrandPre, T., Nakamura, F., Vartanian, T. & Strittmatter, S. M. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature 403, 439–444 (2000).
Prinjha, R. et al. Inhibitor of neurite outgrowth in humans. Nature 403, 383–384 (2000).
Kim, J. E., Li, S., GrandPre, T., Qiu, D. & Strittmatter, S. M. Axon regeneration in young adult mice lacking Nogo-A/B. Neuron 38, 187–199 (2003).
Simonen, M. et al. Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-A improves regenerative and plastic responses after spinal cord injury. Neuron 38, 201–211 (2003).
Zheng, B. et al. Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron 38, 213–224 (2003).
Shao, Z. et al. TAJ/TROY, an orphan TNF receptor family member, binds Nogo-66 receptor 1 and regulates axonal regeneration. Neuron 45, 353–359 (2005).
Mi, S. et al. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nature Neurosci. 7, 221–228 (2004).
Wang, K. C., Kim, J. A., Sivasankaran, R., Segal, R. & He, Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature 420, 74–78 (2002).
Barton, W. A. et al. Structure and axon outgrowth inhibitor binding of the Nogo-66 receptor and related proteins. EMBO J. 22, 3291–3302 (2003).
Lauren, J., Airaksinen, M. S., Saarma, M. & Timmusk, T. Two novel mammalian Nogo receptor homologs differentially expressed in the central and peripheral nervous systems. Mol. Cell. Neurosci. 24, 581–594 (2003).
Venkatesh, K. et al. The Nogo-66 receptor homolog NgR2 is a sialic acid-dependent receptor selective for myelin-associated glycoprotein. J. Neurosci. 25, 808–822 (2005).
Livesey, F. J. & Hunt, S. P. Netrin and netrin receptor expression in the embryonic mammalian nervous system suggests roles in retinal, striatal, nigral, and cerebellar development. Mol. Cell. Neurosci. 8, 417–429 (1997).
Wang, H., Copeland, N. G., Gilbert, D. J., Jenkins, N. A. & Tessier-Lavigne, M. Netrin-3, a mouse homolog of human NTN2L, is highly expressed in sensory ganglia and shows differential binding to netrin receptors. J. Neurosci. 19, 4938–4947 (1999).
Manitt, C. et al. Widespread expression of netrin-1 by neurons and oligodendrocytes in the adult mammalian spinal cord. J. Neurosci. 21, 3911–3922 (2001).
Wehrle, R., Camand, E., Chedotal, A., Sotelo, C. & Dusart, I. Expression of netrin-1, slit-1 and slit-3 but not of slit-2 after cerebellar and spinal cord lesions. Eur. J. Neurosci. 22, 2134–2144 (2005).
Gad, J. M., Keeling, S. L., Wilks, A. F., Tan, S. S. & Cooper, H. M. The expression patterns of guidance receptors, DCC and Neogenin, are spatially and temporally distinct throughout mouse embryogenesis. Dev. Biol. 192, 258–273 (1997).
Gad, J. M., Keeling, S. L., Shu, T., Richards, L. J. & Cooper, H. M. The spatial and temporal expression patterns of netrin receptors, DCC and neogenin, in the developing mouse retina. Exp. Eye Res. 70, 711–722 (2000).
Keino-Masu, K. et al. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell 87, 175–185 (1996).
Engelkamp, D. Cloning of three mouse Unc5 genes and their expression patterns at mid-gestation. Mech. Dev. 118, 191–197 (2002).
Zhang, J. H., Cerretti, D. P., Yu, T., Flanagan, J. G. & Zhou, R. Detection of ligands in regions anatomically connected to neurons expressing the Eph receptor Bsk: potential roles in neuron–target interaction. J. Neurosci. 16, 7182–7192 (1996).
O'Leary, D. D. & McLaughlin, T. Mechanisms of retinotopic map development: Ephs, ephrins, and spontaneous correlated retinal activity. Prog. Brain Res. 147, 43–65 (2005).
Rodger, J. et al. Eph/ephrin expression in the adult rat visual system following localized retinal lesions: localized and transneuronal up-regulation in the retina and superior colliculus. Eur. J. Neurosci. 22, 1840–1852 (2005).
Liebl, D. J., Morris, C. J., Henkemeyer, M. & Parada, L. F. mRNA expression of ephrins and Eph receptor tyrosine kinases in the neonatal and adult mouse central nervous system. J. Neurosci. Res. 71, 7–22 (2003). A comprehensive comparison of ephrin/Eph expression in the neonatal versus adult mouse CNS.
Mori, T., Wanaka, A., Taguchi, A., Matsumoto, K. & Tohyama, M. Differential expressions of the eph family of receptor tyrosine kinase genes (sek, elk, eck) in the developing nervous system of the mouse. Brain Res. Mol. Brain Res. 29, 325–335 (1995).
Irizarry-Ramirez, M. et al. Upregulation of EphA3 receptor after spinal cord injury. J. Neurotrauma 22, 929–935 (2005).
Martone, M. E., Holash, J. A., Bayardo, A., Pasquale, E. B. & Ellisman, M. H. Immunolocalization of the receptor tyrosine kinase EphA4 in the adult rat central nervous system. Brain Res. 771, 238–250 (1997).
Moreno-Flores, M. T. & Wandosell, F. Up-regulation of Eph tyrosine kinase receptors after excitotoxic injury in adult hippocampus. Neuroscience 91, 193–201 (1999).
Goldshmit, Y., Galea, M. P., Wise, G., Bartlett, P. F. & Turnley, A. M. Axonal regeneration and lack of astrocytic gliosis in EphA4-deficient mice. J. Neurosci. 24, 10064–10073 (2004).
Fabes, J. et al. Accumulation of the inhibitory receptor EphA4 may prevent regeneration of corticospinal tract axons following lesion. Eur. J. Neurosci. 23, 1721–1730 (2006).
Liu, X., Hawkes, E., Ishimaru, T., Tran, T. & Sretavan, D. W. EphB3: an endogenous mediator of adult axonal plasticity and regrowth after CNS injury. J. Neurosci. 26, 3087–3101 (2006).
Zhou, R. The Eph family receptors and ligands. Pharmacol. Ther. 77, 151–181 (1998).
Bundesen, L. Q., Scheel, T. A., Bregman, B. S. & Kromer, L. F. Ephrin-B2 and EphB2 regulation of astrocyte-meningeal fibroblast interactions in response to spinal cord lesions in adult rats. J. Neurosci. 23, 7789–7800 (2003).
Holmes, G. P. et al. Distinct but overlapping expression patterns of two vertebrate slit homologs implies functional roles in CNS development and organogenesis. Mech. Dev. 79, 57–72 (1998).
Marillat, V. et al. Spatiotemporal expression patterns of slit and robo genes in the rat brain. J. Comp. Neurol. 442, 130–155 (2002). An extensively detailed anatomical survey.
Sundaresan, V. et al. Dynamic expression patterns of Robo (Robo1 and Robo2) in the developing murine central nervous system. J. Comp. Neurol. 468, 467–481 (2004).
Marillat, V. et al. The slit receptor Rig-1/Robo3 controls midline crossing by hindbrain precerebellar neurons and axons. Neuron 43, 69–79 (2004).
Chedotal, A. et al. Semaphorins III and IV repel hippocampal axons via two distinct receptors. Development 125, 4313–4323 (1998).
Kolodkin, A. L. et al. Neuropilin is a semaphorin III receptor. Cell 90, 753–762 (1997).
Pasterkamp, R. J., Anderson, P. N. & Verhaagen, J. Peripheral nerve injury fails to induce growth of lesioned ascending dorsal column axons into spinal cord scar tissue expressing the axon repellent Semaphorin3A. Eur. J. Neurosci. 13, 457–471 (2001).
Pasterkamp, R. J. et al. Expression of the gene encoding the chemorepellent semaphorin III is induced in the fibroblast component of neural scar tissue formed following injuries of adult but not neonatal CNS. Mol. Cell. Neurosci. 13, 143–166 (1999).
Giger, R. J., Pasterkamp, R. J., Heijnen, S., Holtmaat, A. J. & Verhaagen, J. Anatomical distribution of the chemorepellent semaphorin III/collapsin-1 in the adult rat and human brain: predominant expression in structures of the olfactory-hippocampal pathway and the motor system. J. Neurosci. Res. 52, 27–42 (1998).
De Winter, F. et al. Injury-induced class 3 semaphorin expression in the rat spinal cord. Exp. Neurol. 175, 61–75 (2002).
Sahay, A. et al. Secreted semaphorins modulate synaptic transmission in the adult hippocampus. J. Neurosci. 25, 3613–3620 (2005).
Kawakami, A., Kitsukawa, T., Takagi, S. & Fujisawa, H. Developmentally regulated expression of a cell surface protein, neuropilin, in the mouse nervous system. J. Neurobiol. 29, 1–17 (1996).
Tamagnone, L. et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 99, 71–80 (1999).
Moreau-Fauvarque, C. et al. The transmembrane semaphorin Sema4D/CD100, an inhibitor of axonal growth, is expressed on oligodendrocytes and upregulated after CNS lesion. J. Neurosci. 23, 9229–9239 (2003).
Schmidtmer, J. & Engelkamp, D. Isolation and expression pattern of three mouse homologues of chick Rgm. Gene Expr. Patterns 4, 105–110 (2004).
Oldekamp, J., Kramer, N., Alvarez-Bolado, G. & Skutella, T. Expression pattern of the repulsive guidance molecules RGM A, B and C during mouse development. Gene Expr. Patterns 4, 283–288 (2004).
Keeling, S. L., Gad, J. M. & Cooper, H. M. Mouse Neogenin, a DCC-like molecule, has four splice variants and is expressed widely in the adult mouse and during embryogenesis. Oncogene 15, 691–700 (1997).
Barth, M., Hirsch, H. V., Meinertzhagen, I. A. & Heisenberg, M. Experience-dependent developmental plasticity in the optic lobe of Drosophila melanogaster. J. Neurosci. 17, 1493–1504 (1997).
Brenowitz, E. A. & Beecher, M. D. Song learning in birds: diversity and plasticity, opportunities and challenges. Trends Neurosci. 28, 127–132 (2005).
Gordon, J. A. & Stryker, M. P. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J. Neurosci. 16, 3274–3286 (1996).
Issa, N. P., Trachtenberg, J. T., Chapman, B., Zahs, K. R. & Stryker, M. P. The critical period for ocular dominance plasticity in the ferret's visual cortex. J. Neurosci. 19, 6965–6978 (1999).
Olson, C. R. & Freeman, R. D. Profile of the sensitive period for monocular deprivation in kittens. Exp. Brain Res. 39, 17–21 (1980).
Banks, M. S., Aslin, R. N. & Letson, R. D. Sensitive period for the development of human binocular vision. Science 190, 675–677 (1975).
Berardi, N., Pizzorusso, T. & Maffei, L. Critical periods during sensory development. Curr. Opin. Neurobiol. 10, 138–145 (2000).
Acknowledgements
We thank members of the Strittmatter laboratory for critical discussions, especially B. P. Liu, S. O. Budel, W. B. Cafferty, Y. S. Yang, A. W. McGee, J. H. Park and E. C. Gunther for their extensive comments on this manuscript. J. B. Carmel also provided very helpful suggestions. This work was supported by grants from the National Institute of Neurological Disorders and Stroke (NINDS), from the Christopher Reeve Paralysis Foundation and from the Falk Medical Research Trust (S.M.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Related links
Glossary
- Morphogens
-
Diffusible proteins that are involved in signalling the differentiation of cells into specific tissues and organs during embryogenesis. More recently, they have also been shown to have roles in axon guidance.
- Radial glia
-
Progenitor cell type that gives rise to immature neurons and other radial glia. Immature neurons then migrate along radial glial processes.
- Extracellular matrix
-
(ECM). Connective tissue produced largely by fibroblasts and astrocytes that provides diverse inhibitory and growth-promoting signals to neurons and their extensions.
- Experience-dependent plasticity
-
The reorganization of neural circuits in response to excitatory and inhibitory synaptic influences. Involved in learning and adaptation to varying external stimuli.
- Critical periods
-
Discrete phases early in life during which neural circuits exhibit maximal experience-dependent plasticity.
- Ocular dominance
-
Neurons in the visual cortex respond electrophysiologically to light stimuli from one eye to a greater extent than to stimuli from the other eye. A model system for studying plasticity.
- Monocular deprivation
-
Experimental model in which one eye is sutured shut during the critical period for ocular dominance plasticity, preventing experience-dependent changes.
- Central pattern generators
-
(CPGs). Local circuits involved in coordinating largely automatic motor behaviours such as ambulation and swimming. Modulated by sensory feedback and descending voluntary inputs.
- Chondroitin sulphate proteoglycans
-
(CSPGs).Carbohydrate-rich extracellular molecules with inhibitory effects on neurite outgrowth. Produced predominantly by astrocytes.
- Myelin-associated inhibitors
-
(MAIs). Surface proteins expressed by oligodendrocytes that prevent neurite outgrowth or regeneration.
- Regeneration-associated genes
-
Genes that are upregulated following axonal injury (for example, Gap43, Sprr 1a, Fn14 and arginase I). Increased expression correlates with regeneration in peripheral but not central neurons.
- Body-weight-supported treadmill training
-
(BWSTT). Physical therapy technique for SCI patient's using a harness to partially support the patients weight while therapists assist the patient's legs to ambulate on a moving treadmill.
Rights and permissions
About this article
Cite this article
Harel, N., Strittmatter, S. Can regenerating axons recapitulate developmental guidance during recovery from spinal cord injury?. Nat Rev Neurosci 7, 603–616 (2006). https://doi.org/10.1038/nrn1957
Issue Date:
DOI: https://doi.org/10.1038/nrn1957