Key Points
-
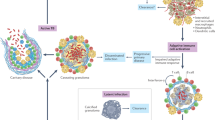
Latent tuberculosis is defined by the presence of an antigen-specific T cell response in the absence of clinical symptoms. It includes conditions ranging from clearance of the pathogen, maintenance of quiescent infected foci to active subclinical disease, and can be viewed as part of a spectrum of outcomes to infection with Mycobacterium tuberculosis.
-
The pathogenesis of tuberculosis depends on localized granulomatous responses. Different types of granuloma can promote bacterial killing, persistence or replication.
-
Live imaging by positron emission tomography and computed tomography in humans and non-human primates provides powerful new opportunities to analyse tuberculosis lesions in terms of spatial distribution and dynamic behaviour in the presence and absence of drugs.
-
Different granulomas provide different microenvironments that can support heterogeneous subpopulations of bacteria differing in phenotypic adaptation and drug susceptibility. Systems biology approaches will be important in the molecular characterization of the microenvironment within lesions and the corresponding bacterial physiology.
-
Drug strategies based on simple inhibition of individual enzyme targets have proved largely ineffective for the discovery of novel antimicrobials. An improved understanding of actual bactericidal mechanisms will be important in rational targeting of new drugs against persistent bacterial phenotypes.
-
In addition to the development of drugs that are customized against different subpopulations of bacteria, the use of preventive therapy as an intervention for the control of global tuberculosis will benefit from further definition of the spectrum of latent disease. Imaging profiles and biomarkers will assist in clinical trials of new drugs and in the identification of individuals at highest risk disease progression.
Abstract
Immunological tests provide evidence of latent tuberculosis in one third of the global population, which corresponds to more than two billion individuals. Latent tuberculosis is defined by the absence of clinical symptoms but carries a risk of subsequent progression to clinical disease, particularly in the context of co-infection with HIV. In this Review we discuss the biology of latent tuberculosis as part of a broad range of responses that occur following infection with Mycobacterium tuberculosis, which result in the formation of physiologically distinct granulomatous lesions that provide microenvironments with differential ability to support or suppress the persistence of viable bacteria. We then show how this model can be used to develop a rational programme to discover effective drugs for the eradication of M. tuberculosis infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Comstock, G. W., Baum, C. & Snider, D. E. Jr. Isoniazid prophylaxis among Alaskan Eskimos: a final report of the bethel isoniazid studies. Am. Rev. Respir. Dis. 119, 827–830 (1979).
Dye, C. & Williams, B. G. Eliminating human tuberculosis in the twenty-first century. J. R. Soc. Interface 5, 653–662 (2008).
Stead, W. W. Management of health care workers after inadvertent exposure to tuberculosis: a guide for the use of preventive therapy. Ann. Intern. Med. 122, 906–912 (1995).
Pai, M., Zwerling, A. & Menzies, D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann. Intern. Med. 149, 177–184 (2008).
Young, D. B., Gideon, H. P. & Wilkinson, R. J. Eliminating latent tuberculosis. Trends Microbiol. 17, 193–188 (2009).
Mtei, L. et al. High rates of clinical and subclinical tuberculosis among HIV-infected ambulatory subjects in Tanzania. Clin. Infect. Dis. 40, 1500–1507 (2005).
Vandiviere, H. M., Loring, W. E., Melvin, I. & Willis, S. The treated pulmonary lesion and its tubercle bacillus. II. The death and resurrection. Am. J. Med. Sci. 232, 30–37 (1956).
Capuano, S. V. 3rd et al. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect. Immun. 71, 5831–5844 (2003).
Lin, P. L. et al. Early events in Mycobacterium tuberculosis infection in cynomolgus macaques. Infect. Immun. 74, 3790–3803 (2006).
Goo, J. M. et al. Pulmonary tuberculoma evaluated by means of FDG PET: findings in 10 cases. Radiology 216, 117–121 (2000).
Hara, T., Kosaka, N., Suzuki, T., Kudo, K. & Niino, H. Uptake rates of 18F-fluorodeoxyglucose and 11C-choline in lung cancer and pulmonary tuberculosis: a positron emission tomography study. Chest 124, 893–901 (2003).
Yang, C. M., Hsu, C. H., Lee, C. M. & Wang, F. C. Intense uptake of [F-18]-fluoro-2 deoxy-D-glucose in active pulmonary tuberculosis. Ann. Nucl. Med. 17, 407–410 (2003).
Park, I. N., Ryu, J. S. & Shim, T. S. Evaluation of therapeutic response of tuberculoma using F-18 FDG positron emission tomography. Clin. Nucl. Med. 33, 1–3 (2008).
Canetti, G., Parrot, R., Porven, G. & Le Lirzin, M. Rifamycin levels in the lung and tuberculous lesions in man. Acta Tuberc. Pneumol. Belg. 60, 315–322 (1969).
Kislitsyna, N. A. Comparative evaluation of rifampicin and isoniazid penetration into the pathological foci of the lungs in tuberculosis patients. Probl. Tuberk. 55–57 (1985).
Kislitsyna, N. A. & Kotova, N. I. Rifampicin and isoniazid concentration in the blood and resected lungs in tuberculosis with combined use of the preparations. Probl. Tuberk 8, 63–65 (1980).
Sauermann, R. et al. Antibiotic abscess penetration: fosfomycin levels measured in pus and simulated concentration-time profiles. Antimicrob. Agents Chemother. 49, 4448–4454 (2005).
Wagner, C., Sauermann, R. & Joukhadar, C. Principles of antibiotic penetration into abscess fluid. Pharmacology 78, 1–10 (2006).
Cotran, R. S., Kumar, V. & Robbins, S. L. in Pathologic Basis of Disease (ed. Company, W. B. S.) (Saunders, Philadelphia, 1989).
Dannenberg, A. M. Jr in Pathogenesis of Human Pulmonary Tuberculosis 36–64 (ASM, Washington D. C., 2006). A comprehensive review of five decades of literature on the pathogenesis of tuberculosis in humans and in the rabbit model, including comparisons with other animal models.
Via, L. E. et al. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect. Immun. 76, 2333–2340 (2008). A conclusive demonstration that hypoxia is a relevant phenotype in several non-mouse animal models of TB.
Rhoades, E. R., Frank, A. A. & Orme, I. M. Progression of chronic pulmonary tuberculosis in mice aerogenically infected with virulent Mycobacterium tuberculosis. Tuber Lung Dis. 78, 57–66 (1997).
Radaeva, T. V., Nikonenko, B. V., Mischenko, V. V., Averbakh, M. M. Jr & Apt, A. S. Direct comparison of low-dose and Cornell-like models of chronic and reactivation tuberculosis in genetically susceptible I/St and resistant B6 mice. Tuberculosis (Edinb.) 85, 65–72 (2005).
Sissons, J. et al. Multigenic control of tuberculosis resistance: analysis of a QTL on mouse chromosome 7 and its synergism with sst1. Genes Immun. 10, 37–46 (2009).
Manabe, Y. C. et al. The aerosol rabbit model of TB latency, reactivation and immune reconstitution inflammatory syndrome. Tuberculosis (Edinb.) 88, 187–196 (2008).
Kesavan, A. K., Brooks, M., Tufariello, J., Chan, J. & Manabe, Y. C. Tuberculosis genes expressed during persistence and reactivation in the resistant rabbit model. Tuberculosis (Edinb.) 89, 17–21 (2009).
Tsenova, L. et al. Virulence of selected Mycobacterium tuberculosis clinical isolates in the rabbit model of meningitis is dependent on phenolic glycolipid produced by the bacilli. J. Infect. Dis. 192, 98–106 (2005).
Gandotra, S., Schnappinger, D., Monteleone, M., Hillen, W. & Ehrt, S. In vivo gene silencing identifies the Mycobacterium tuberculosis proteasome as essential for the bacteria to persist in mice. Nature Med. 13, 1515–1520 (2007). Demonstration of the use of conditional gene expression systems to study mycobacterial pathogenesis.
Sonnenberg, P. et al. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J. Infect. Dis. 191, 150–158 (2005).
Keane, J. et al. Tuberculosis associated with infliximab, a tumor necrosis factor α-neutralizing agent. N. Engl. J. Med. 345, 1098–1104 (2001). The first demonstration that suppressing TNF levels correlates with reactivation of latent TB, the implication being that there is a delicate balance between immune function and the development of disease.
Marino, S. et al. Differences in reactivation of tuberculosis induced from anti-TNF treatments are based on bioavailability in granulomatous tissue. PLoS Comput. Biol. 3, 1909–1924 (2007).
Sud, D., Bigbee, C., Flynn, J. L. & Kirschner, D. E. Contribution of CD8+ T cells to control of Mycobacterium tuberculosis infection. J. Immunol. 176, 4296–4314 (2006).
Ray, J. C., Flynn, J. L. & Kirschner, D. E. Synergy between individual TNF-dependent functions determines granuloma performance for controlling Mycobacterium tuberculosis infection. J. Immunol. 182, 3706–3717 (2009). Illustrates the use of modelling to understand the complex dynamics of tuberculous granulomas.
Timm, J. et al. Differential expression of iron-, carbon-, and oxygen-responsive mycobacterial genes in the lungs of chronically infected mice and tuberculosis patients. Proc. Natl Acad. Sci. USA 100, 14321–14326 (2003). This is the first study to attempt to relate the transcriptional responses in humans to conditions relevant to disease.
Fenhalls, G. et al. In situ detection of Mycobacterium tuberculosis transcripts in human lung granulomas reveals differential gene expression in necrotic lesions. Infect. Immun. 70, 6330–6338 (2002).
Xie, Z., Siddiqi, N. & Rubin, E. J. Differential antibiotic susceptibilities of starved Mycobacterium tuberculosis isolates. Antimicrob. Agents Chemother. 49, 4778–4780 (2005).
Paramasivan, C. N., Sulochana, S., Kubendiran, G., Venkatesan, P. & Mitchison, D. A. Bactericidal action of gatifloxacin, rifampin, and isoniazid on logarithmic- and stationary-phase cultures of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 49, 627–631 (2005).
Herbert, D. et al. Bactericidal action of ofloxacin, sulbactam-ampicillin, rifampin, and isoniazid on logarithmic- and stationary-phase cultures of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 40, 2296–2299 (1996).
Wayne, L. G. & Sramek, H. A. Metronidazole is bactericidal to dormant cells of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 38, 2054–2058 (1994).
Schnappinger, D. et al. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: insights into the phagosomal environment. J. Exp. Med. 198, 693–704 (2003).
Rohde, K. H., Abramovitch, R. B. & Russell, D. G. Mycobacterium tuberculosis invasion of macrophages: linking bacterial gene expression to environmental cues. Cell Host Microbe 2, 352–364 (2007).
Tailleux, L. et al. Probing host pathogen cross-talk by transcriptional profiling of both Mycobacterium tuberculosis and infected human dendritic cells and macrophages. PLoS ONE 3, e1403 (2008).
Talaat, A. M. et al. Mycobacterial bacilli are metabolically active during chronic tuberculosis in murine lungs: insights from genome-wide transcriptional profiling. J. Bacteriol. 189, 4265–4274 (2007).
Voskuil, M. I. et al. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J. Exp. Med. 198, 705–713 (2003).
Rodriguez, G. M., Voskuil, M. I., Gold, B., Schoolnik, G. K. & Smith, I. ideR, an essential gene in Mycobacterium tuberculosis: role of IdeR in iron-dependent gene expression, iron metabolism, and oxidative stress response. Infect. Immun. 70, 3371–3381 (2002).
Fisher, M. A., Plikaytis, B. B. & Shinnick, T. M. Microarray analysis of the Mycobacterium tuberculosis transcriptional response to the acidic conditions found in phagosomes. J. Bacteriol. 184, 4025–4032 (2002).
Rustad, T. R., Harrell, M. I., Liao, R. & Sherman, D. R. The enduring hypoxic response of Mycobacterium tuberculosis. PLoS ONE 3, e1502 (2008).
Converse, P. J. et al. Role of the dosR-dosS two-component regulatory system in Mycobacterium tuberculosis virulence in three animal models. Infect. Immun. 77, 1230–1237 (2009).
Malhotra, V. et al. Disruption of response regulator gene, devR, leads to attenuation in virulence of Mycobacterium tuberculosis. FEMS Microbiol. Lett. 231, 237–245 (2004).
Dahl, J. L. et al. The relA homolog of Mycobacterium smegmatis affects cell appearance, viability, and gene expression. J. Bacteriol. 187, 2439–2447 (2005).
McKinney, J. D. et al. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406, 735–738 (2000).
Munoz-Elias, E. J., Upton, A. M., Cherian, J. & McKinney, J. D. Role of the methylcitrate cycle in Mycobacterium tuberculosis metabolism, intracellular growth, and virulence. Mol. Microbiol 60, 1109–1122 (2006).
Rachman, H. et al. Unique transcriptome signature of Mycobacterium tuberculosis in pulmonary tuberculosis. Infect. Immun. 74, 1233–1242 (2006).
Garton, N. J. et al. Cytological and transcript analyses reveal fat and lazy persister-like bacilli in tuberculous sputum. PLoS Med. 5, e75 (2008). This work raises new questions about the metabolic consequences of activation of the dormancy regulon in M. tuberculosis and the presence of lipid bodies.
Boshoff, H. I. et al. Biosynthesis and recycling of nicotinamide cofactors in Mycobacterium tuberculosis. An essential role for NAD in nonreplicating bacilli. J. Biol. Chem. 283, 19329–19341 (2008).
Sassetti, C. M. & Rubin, E. J. Genetic requirements for mycobacterial survival during infection. Proc. Natl Acad. Sci. USA 100, 12989–12994 (2003).
Beste, D. J. et al. GSMN-TB: a web-based genome-scale network model of Mycobacterium tuberculosis metabolism. Genome Biol. 8, R89 (2007).
Raman, K., Rajagopalan, P. & Chandra, N. Flux balance analysis of mycolic acid pathway: targets for anti-tubercular drugs. PLoS Comput. Biol. 1, e46 (2005).
Bloch, H. & Segal, W. Biochemical differentiation of Mycobacterium tuberculosis grown in vivo and in vitro. J. Bacteriol. 72, 132–141 (1956).
Segal, W. & Bloch, H. Pathogenic and immunogenic differentiation of Mycobacterium tuberculosis grown in vitro and in vivo. Am. Rev. Tuberc. 75, 495–500 (1957).
Lange, R. P., Locher, H. H., Wyss, P. C. & Then, R. L. The targets of currently used antibacterial agents: lessons for drug discovery. Curr. Pharm. Des 13, 3140–3154 (2007).
Walsh, C. in Antibiotics: Actions, Origins, Resistance 11–88 (ASM, Washington, D. C., 2003).
Kohanski, M. A., Dwyer, D. J., Wierzbowski, J., Cottarel, G. & Collins, J. J. Mistranslation of membrane proteins and two-component system activation trigger antibiotic-mediated cell death. Cell 135, 679–690 (2008). A must-read illustration of the complexities of the mechanism by which antibiotics cause bacteria to die.
Kohanski, M. A., Dwyer, D. J., Hayete, B., Lawrence, C. A. & Collins, J. J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130, 797–810 (2007).
Singh, R. et al. PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release. Science 322, 1392–1395 (2008). Identification of new mycobactericidal mechanism under anaerobic conditions.
Rao, S. P., Alonso, S., Rand, L., Dick, T. & Pethe, K. The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA 105, 11945–11950 (2008).
Dhar, N. & McKinney, J. D. Microbial phenotypic heterogeneity and antibiotic tolerance. Curr. Opin. Microbiol. 10, 30–38 (2007).
Brehm-Stecher, B. F. & Johnson, E. A. Single-cell microbiology: tools, technologies, and applications. Microbiol. Mol. Biol. Rev. 68, 538–559 (2004).
Payne, D. J., Gwynn, M. N., Holmes, D. J. & Pompliano, D. L. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nature Rev. Drug Discov. 6, 29–40 (2007). A review that describes many of the challenges complicating antibacterial drug development.
Silver, L. L. Multi-targeting by monotherapeutic antibacterials. Nature Rev. Drug Discov. 6, 41–55 (2007).
Manjunatha, U. H. et al. Identification of a nitroimidazo-oxazine-specific protein involved in PA-824 resistance in Mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA 103, 431–436 (2006).
Andries, K. et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307, 223–227 (2005). First report of a promising new antimycobacterial agent with activity against replicating and non-replicating cultures.
Terstappen, G. C., Schlupen, C., Raggiaschi, R. & Gaviraghi, G. Target deconvolution strategies in drug discovery. Nature Rev. Drug Discov. 6, 891–903 (2007).
Boshoff, H. I. et al. The transcriptional responses of Mycobacterium tuberculosis to inhibitors of metabolism: novel insights into drug mechanisms of action. J. Biol. Chem. 279, 40174–40184 (2004).
Dartois V., Leong, F. J. & Dick, T. in Antiparasitic and Antibacterial Drug Discovery: From Molecular Targets to Drug Candidates. (ed. Seltzer, P. M.) 415–440 (Wiley-VCH, Weinheim, 2009).
Ciulli, A. & Abell, C. Fragment-based approaches to enzyme inhibition. Curr. Opin. Biotechnol. 18, 489–496 (2007).
Nikaido, H. & Jarlier, V. Permeability of the mycobacterial cell wall. Res. Microbiol 142, 437–443 (1991).
Liu, J., Barry, C. E. 3rd, Besra, G. S. & Nikaido, H. Mycolic acid structure determines the fluidity of the mycobacterial cell wall. J. Biol. Chem. 271, 29545–29551 (1996).
Faller, M., Niederweis, M. & Schulz, G. E. The structure of a mycobacterial outer-membrane channel. Science 303, 1189–1192 (2004).
Sassetti, C. M., Boyd, D. H. & Rubin, E. J. Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 48, 77–84 (2003).
Pichota, A. et al. Peptide deformylase inhibitors of Mycobacterium tuberculosis: synthesis, structural investigations, and biological results. Bioorg. Med. Chem. Lett. 18, 6568–6572 (2008).
Klotzsche, M., Ehrt, S. & Schnappinger, D. Improved tetracycline repressors for gene silencing in mycobacteria. Nucleic Acids Res. 37, 1778–1788 (2009).
Ferebee, S. H. & Mount, F. W. Tuberculosis morbidity in a controlled trial of the prophylactic use of isoniazid among household contacts. Am. Rev. Respir. Dis. 85, 490–510 (1962).
Ferebee, S. H., Mount, F. W., Murray, F. J. & Livesay, V. T. A controlled trial of isoniazid prophylaxis in mental institutions. Am. Rev. Respir. Dis. 88, 161–175 (1963).
Wallis, R. S. et al. Biomarkers for tuberculosis disease activity, cure, and relapse. Lancet Infect. Dis. 9, 162–172 (2009).
Ewer, K. et al. Dynamic antigen-specific T-cell responses after point-source exposure to Mycobacterium tuberculosis. Am. J. Respir. Crit. Care Med. 174, 831–839 (2006).
Goletti, D. et al. Isoniazid prophylaxis differently modulates T-cell responses to RD1-epitopes in contacts recently exposed to Mycobacterium tuberculosis: a pilot study. Respir. Res. 8, 5 (2007).
Wilkinson, K. A. et al. Effect of treatment of latent tuberculosis infection on the T cell response to Mycobacterium tuberculosis antigens. J. Infect. Dis. 193, 354–359 (2006).
Higuchi, K., Harada, N. & Mori, T. Interferon-γ responses after isoniazid chemotherapy for latent tuberculosis. Respirology 13, 468–472 (2008).
Veening, G. J. Long term isoniazid prophylaxis. Controlled trial on INH prophylaxis after recent tuberculin conversion in young adults. Bull. Int. Union Tuberc. 41, 169–171 (1968).
Comstock, G. W. & Woolpert, S. F. in The Mycobacteria: A Sourcebook (eds Kubica, G. P. & Wayne, L. G.) 1071–1082 (Marcel Dekker, New York, 1984).
Lin, P. L. et al. Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect Immun. 77, 4631–4642 (2009).
Acknowledgements
We are grateful to colleagues in the Grand Challenges in Global Health project “Drugs for Treatment of Latent Tuberculosis” for stimulating discussion and to the Bill and Melinda Gates Foundation and the Wellcome Trust for financial support. This work was also funded (in part) by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID) and by NIH RO1 HL075845 (J.L.F.). We also thank our Novartis colleagues in Singapore, Basel, Cambridge and La Jolla for discussions and collaboration.
Author information
Authors and Affiliations
Corresponding author
Related links
Glossary
- Tuberculin skin test
-
A method of diagnosing Mycobacterium tuberculosis infection by injecting tuberculosis antigens intradermally. A delayed type hypersensitivity response, dependent on the presence of sensitized T cells, is seen in those infected with M. tuberculosis. This does not distinguish latent infection from active tuberculosis.
- Purified protein derivative
-
A precipitate of non-specific molecules from sterilized and filtered cultures of Mycobacterium tuberculosis.
- Computed tomography
-
An imaging technique in which X-ray scans of a subject are compiled to generate a three-dimensional picture of various organs and structures (including brain and lungs).
- Positron emission tomography
-
A nuclear medicine imaging technique using a positron-emitting probe that produces a three-dimensional image of biological processes within the scanned subject.
- Granuloma
-
An organized structure comprising lymphocytes, macrophages, neutrophils and sometimes fibroblasts that often has a necrotic centre, which arises in response to continued antigenic stimulation in the presence of macrophages, for example in response to Mycobacterium tuberculosis infection.
- Hypoxia
-
Low levels of oxygen in tissues.
- Paucibacillary
-
Containing just a few bacilli, as is the case with granulomas that are caused by various mycobacterial diseases.
- Caseation
-
Necrotic degeneration of bodily tissue into a soft crumbly cheese-like mass, where cellular outline is lost. In tuberculosis, this refers to the necrotic centre of a granuloma and is mainly driven by the immune response and is typical of tuberculosis pathogenesis.
- Hypoxic response
-
Changes in gene expression induced by the incubation of Mycobacterium tuberculosis under anaerobic conditions. This comprises an initial transient response (by the DosR hypoxia regulon) followed by the enduring hypoxic response involving expression of a set of 230 genes.
- Hypoxia regulon
-
A cluster of 48 genes that are controlled by the transcriptional regulator DosR, which is upregulated in response to hypoxia but also during exposure to nitric oxide, carbon monoxide, sodium dodecyl sulfate or low pH.
Rights and permissions
About this article
Cite this article
Barry, C., Boshoff, H., Dartois, V. et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol 7, 845–855 (2009). https://doi.org/10.1038/nrmicro2236
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro2236