Key Points
-
Flaviviruses and hepaciviruses share similarities in their fundamental replication mechanisms and strategies to manipulate the host cell, yet important differences exist, likely reflecting the use of distinct host cell pathways.
-
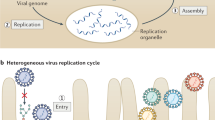
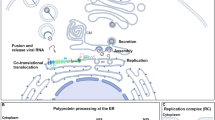
RNA replication of Flaviviridae family members occurs in tight association with endoplasmic reticulum-derived membranes, which are reorganized into viral replication organelles. Whereas the morphology and the architecture of these replication organelles are well defined, relatively little is known about the viral and cellular factors orchestrating their biogenesis.
-
Protein folding, modification and degradation are essential, tightly regulated cellular processes, and a number of common host factors and pathways that are involved in these processes appear to be exploited by both flaviviruses and hepaciviruses at different steps of their replication cycle. These include heat shock protein 70 (HSP70) network components, the unfolded protein response, the ubiquitin-dependent proteasome system and autophagy.
-
Accumulating evidence indicates that lipids and lipid metabolism fulfil essential roles in the life cycle of Flaviviridae viruses. They alter the lipid composition of cellular membranes, serving as scaffold for the assembly of the viral replicase by changing their biophysical properties, such as curvature, permeability and fluidity.
-
The identification of host cell pathways and factors commonly used by members of the Flaviviridae family might help in the development of broad-spectrum antiviral drugs that target multiple members of this family and/or other virus families.
-
As exemplified by members of the Flaviviridae family, the use of host cell pathways does not follow conventional phylogeny but, rather, reveals unexpected commonalities with distantly related viruses, raising the question of evolutionary relationships between these viruses.
Abstract
Members of the Flaviviridae virus family comprise a large group of enveloped viruses with a single-strand RNA genome of positive polarity. Several genera belong to this family, including the Hepacivirus genus, of which hepatitis C virus (HCV) is the prototype member, and the Flavivirus genus, which contains both dengue virus and Zika virus. Viruses of these genera differ in many respects, such as the mode of transmission or the course of infection, which is either predominantly persistent in the case of HCV or acutely self-limiting in the case of flaviviruses. Although the fundamental replication strategy of Flaviviridae members is similar, during the past few years, important differences have been discovered, including the way in which these viruses exploit cellular resources to facilitate viral propagation. These differences might be responsible, at least in part, for the various biological properties of these viruses, thus offering the possibility to learn from comparisons. In this Review, we discuss the current understanding of how Flaviviridae viruses manipulate and usurp cellular pathways in infected cells. Specifically, we focus on comparing strategies employed by flaviviruses with those employed by hepaciviruses, and we discuss the importance of these interactions in the context of viral replication and antiviral therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
[No authors listed.] Taxonomy. International Committee on Taxonomy of Viruses (ICTV) https://talk.ictvonline.org/taxonomy/ (2017).
[No authors listed.] Hepatitis C fact sheet. World Health Organization http://www.who.int/mediacentre/factsheets/fs164/en/ (2017).
Manns, M. P. et al. Hepatitis C virus infection. Nat. Rev. Dis. Primers. 3, 17006 (2017).
Stanaway, J. D. et al. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect. Dis. 16, 712–723 (2016).
Hadinegoro, S. R. et al. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N. Engl. J. Med. 373, 1195–1206 (2015).
Wikan, N. & Smith, D. R. Zika virus: history of a newly emerging arbovirus. Lancet Infect. Dis. 16, e119–e126 (2016).
Miner, J. J. & Diamond, M. S. Zika virus pathogenesis and tissue tropism. Cell Host Microbe 21, 134–142 (2017).
Paul, D. & Bartenschlager, R. Flaviviridae replication organelles: oh, what a tangled web we weave. Annu. Rev. Virol. 2, 289–310 (2015).
Welsch, S. et al. Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell Host Microbe 5, 365–375 (2009). This study reveals the three-dimensional architecture of DENV ROs using electron tomography. This study also identifies assembling virus particles in close apposition of viral replication sites and provides a model on the spatio-temporal coupling of the different steps of the DENV life cycle.
Cortese, M. et al. Ultrastructural characterization of Zika virus replication factories. Cell Rep. 18, 2113–2123 (2017).
Gillespie, L. K., Hoenen, A., Morgan, G. & Mackenzie, J. M. The endoplasmic reticulum provides the membrane platform for biogenesis of the flavivirus replication complex. J. Virol. 84, 10438–10447 (2010).
Westaway, E. G., Mackenzie, J. M., Kenney, M. T., Jones, M. K. & Khromykh, A. A. Ultrastructure of Kunjin virus-infected cells: colocalization of NS1 and NS3 with double-stranded RNA, and of NS2B with NS3, in virus-induced membrane structures. J. Virol. 71, 6650–6661 (1997).
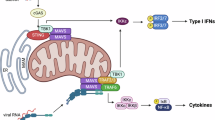
Horner, S. M., Liu, H. M., Park, H. S., Briley, J. & Gale, M. Jr. Mitochondrial-associated endoplasmic reticulum membranes (MAM) form innate immune synapses and are targeted by hepatitis C virus. Proc. Natl Acad. Sci. USA 108, 14590–14595 (2011).
Chatel-Chaix, L. et al. Dengue virus perturbs mitochondrial morphodynamics to dampen innate immune responses. Cell Host Microbe 20, 342–356 (2016).
Junjhon, J. et al. Ultrastructural characterization and three-dimensional architecture of replication sites in dengue virus-infected mosquito cells. J. Virol. 88, 4687–4697 (2014).
Romero-Brey, I. et al. Three-dimensional architecture and biogenesis of membrane structures associated with hepatitis C virus replication. PLOS Pathog. 8, e1003056 (2012). This study uses electron tomography to reveal that HCV ROs are primarily composed of double-membrane vesicles and provides evidence that these structures might be the site of viral RNA replication.
Esser-Nobis, K. et al. Analysis of hepatitis C virus resistance to silibinin in vitro and in vivo points to a novel mechanism involving nonstructural protein 4B. Hepatology 57, 953–963 (2013).
Paul, D., Hoppe, S., Saher, G., Krijnse-Locker, J. & Bartenschlager, R. Morphological and biochemical characterization of the membranous hepatitis C virus replication compartment. J. Virol. 87, 10612–10627 (2013).
Quinkert, D., Bartenschlager, R. & Lohmann, V. Quantitative analysis of the hepatitis C virus replication complex. J. Virol. 79, 13594–13605 (2005).
Miyanari, Y. et al. Hepatitis C virus non-structural proteins in the probable membranous compartment function in viral genome replication. J. Biol. Chem. 278, 50301–50308 (2003).
Neufeldt, C. J. et al. The hepatitis C virus-induced membranous web and associated nuclear transport machinery limit access of pattern recognition receptors to viral replication sites. PLOS Pathog. 12, e1005428 (2016).
Neufeldt, C. J. et al. Hepatitis C virus-induced cytoplasmic organelles use the nuclear transport machinery to establish an environment conducive to virus replication. PLOS Pathog. 9, e1003744 (2013).
Zou, J. et al. Dimerization of flavivirus NS4B protein. J. Virol. 88, 3379–3391 (2014).
Spuul, P. et al. Assembly of alphavirus replication complexes from RNA and protein components in a novel trans-replication system in mammalian cells. J. Virol. 85, 4739–4751 (2011).
Kallio, K., Hellstrom, K., Jokitalo, E. & Ahola, T. RNA replication and membrane modification require the same functions of alphavirus nonstructural proteins. J. Virol. 90, 1687–1692 (2015).
Ertel, K. J. et al. Cryo-electron tomography reveals novel features of a viral RNA replication compartment. eLife 6, e25940 (2017).
Akey, D. L. et al. Flavivirus NS1 structures reveal surfaces for associations with membranes and the immune system. Science 343, 881–885 (2014). This study is the first report of the three-dimensional structure of full-length, glycosylated NS1 from WNV and DENV, revealing distinct domains for membrane association of the dimer and interactions with the immune system.
Brown, W. C. et al. Extended surface for membrane association in Zika virus NS1 structure. Nat. Struct. Mol. Biol. 23, 865–867 (2016).
Apte-Sengupta, S., Sirohi, D. & Kuhn, R. J. Coupling of replication and assembly in flaviviruses. Curr. Opin. Virol. 9, 134–142 (2014).
Tabata, K. et al. Unique requirement for ESCRT factors in flavivirus particle formation on the endoplasmic reticulum. Cell Rep. 16, 2339–2347 (2016).
Romero-Brey, I. et al. NS5A domain 1 and polyprotein cleavage kinetics are critical for induction of double-membrane vesicles associated with hepatitis C virus replication. mBio 6, e00759 (2015).
Egger, D. et al. Expression of hepatitis C virus proteins induces distinct membrane alterations including a candidate viral replication complex. J. Virol. 76, 5974–5984 (2002).
Paul, D. et al. NS4B self-interaction through conserved C-terminal elements is required for the establishment of functional hepatitis C virus replication complexes. J. Virol. 85, 6963–6976 (2011).
Madan, V., Paul, D., Lohmann, V. & Bartenschlager, R. Inhibition of HCV replication by cyclophilin antagonists is linked to replication fitness and occurs by inhibition of membranous web formation. Gastroenterology 146, 1361–1372.e9 (2014).
Reiss, S. et al. Recruitment and activation of a lipid kinase by hepatitis C virus NS5A is essential for integrity of the membranous replication compartment. Cell Host Microbe 9, 32–45 (2011).
Moradpour, D. & Penin, F. Hepatitis C virus proteins: from structure to function. Curr. Top. Microbiol. Immunol. 369, 113–142 (2013).
Berger, C. et al. Daclatasvir-like inhibitors of NS5A block early biogenesis of hepatitis C virus-induced membranous replication factories, independent of RNA replication. Gastroenterology 147, 1094–1105.e25 (2014).
Acosta, E. G., Kumar, A. & Bartenschlager, R. Revisiting dengue virus-host cell interaction: new insights into molecular and cellular virology. Adv. Virus Res. 88, 1–109 (2014).
Bartenschlager, R., Lohmann, V. & Penin, F. The molecular and structural basis of advanced antiviral therapy for hepatitis C virus infection. Nat. Rev. Microbiol. 11, 482–496 (2013).
Stocks, C. E. & Lobigs, M. Signal peptidase cleavage at the flavivirus C-prM junction: dependence on the viral NS2B-3 protease for efficient processing requires determinants in C, the signal peptide, and prM. J. Virol. 72, 2141–2149 (1998).
Amberg, S. M. & Rice, C. M. Mutagenesis of the NS2B-NS3-mediated cleavage site in the flavivirus capsid protein demonstrates a requirement for coordinated processing. J. Virol. 73, 8083–8094 (1999).
Lee, E., Stocks, C. E., Amberg, S. M., Rice, C. M. & Lobigs, M. Mutagenesis of the signal sequence of yellow fever virus prM protein: enhancement of signalase cleavage in vitro is lethal for virus production. J. Virol. 74, 24–32 (2000).
Lobigs, M. & Lee, E. Inefficient signalase cleavage promotes efficient nucleocapsid incorporation into budding flavivirus membranes. J. Virol. 78, 178–186 (2004).
Zhang, R. et al. A CRISPR screen defines a signal peptide processing pathway required by flaviviruses. Nature 535, 164–168 (2016). This genome-wide CRISPR–Cas9-based screen to identify host dependency factors for the Flaviviridae life cycle reveals a strong dependence on the signal peptide processing pathway for the replication of this virus family.
Estoppey, D. et al. The natural product cavinafungin selectively interferes with Zika and dengue virus replication by inhibition of the host signal peptidase. Cell Rep. 19, 451–460 (2017).
Idris, F., Muharram, S. H. & Diah, S. Glycosylation of dengue virus glycoproteins and their interactions with carbohydrate receptors: possible targets for antiviral therapy. Arch. Virol. 161, 1751–1760 (2016).
Hundt, J., Li, Z. & Liu, Q. Post-translational modifications of hepatitis C viral proteins and their biological significance. World J. Gastroenterol. 19, 8929–8939 (2013).
Marceau, C. D. et al. Genetic dissection of Flaviviridae host factors through genome-scale CRISPR screens. Nature 535, 159–163 (2016). This is a genome-wide CRISPR–Cas9-based screen that is used to identify host dependency factors for Flaviviridae virus infection and reveals a non-canonical role for the OST complex in DENV RNA replication.
Lin, D. L. et al. Dengue virus hijacks a noncanonical oxidoreductase function of a cellular oligosaccharyltransferase complex. mBio 8, e00939-17 (2017).
Taguwa, S. et al. Defining Hsp70 subnetworks in dengue virus replication reveals key vulnerability in Flavivirus infection. Cell 163, 1108–1123 (2015). This is a comprehensive study examining the role of HSP70 and its specific co-chaperones for Flavivirus replication. Specifically, this study demonstrates a conserved role of the HSP70 network for all stages of the Flavivirus replication cycle.
Taguwa, S. & Frydman, J. The significance of Hsp70 subnetwork for dengue virus lifecycle. Uirusu 65, 179–186 (2015).
Yi, Z. et al. Identification and characterization of the host protein DNAJC14 as a broadly active flavivirus replication modulator. PLOS Pathog. 7, e1001255 (2011).
Bozzacco, L. et al. Chaperone-assisted protein folding is critical for yellow fever virus NS3/4A cleavage and replication. J. Virol. 90, 3212–3228 (2016).
Gonzalez, O. et al. The heat shock protein inhibitor Quercetin attenuates hepatitis C virus production. Hepatology 50, 1756–1764 (2009).
Peng, Z. G. et al. Small molecular compounds that inhibit hepatitis C virus replication through destabilizing heat shock cognate 70 messenger RNA. Hepatology 52, 845–853 (2010).
Khachatoorian, R. et al. Allosteric heat shock protein 70 inhibitors block hepatitis C virus assembly. Int. J. Antimicrob. Agents 47, 289–296 (2016).
Khachatoorian, R. et al. The NS5A-binding heat shock proteins HSC70 and HSP70 play distinct roles in the hepatitis C viral life cycle. Virology 454–455, 118–127 (2014).
Braga, A. C., Carneiro, B. M., Batista, M. N., Akinaga, M. M. & Rahal, P. Inhibition of hepatitis C virus using siRNA targeted to the virus and Hsp90. Cell Stress Chaperones 22, 113–122 (2017).
Baugh, J. M., Garcia-Rivera, J. A. & Gallay, P. A. Host-targeting agents in the treatment of hepatitis C: a beginning and an end? Antiviral Res. 100, 555–561 (2013).
Jheng, J. R., Ho, J. Y. & Horng, J. T. ER stress, autophagy, and RNA viruses. Front. Microbiol. 5, 388 (2014).
Diwaker, D., Mishra, K. P. & Ganju, L. Effect of modulation of unfolded protein response pathway on dengue virus infection. Acta Biochim. Biophys. Sin. 47, 960–968 (2015).
Smith, J. A. A new paradigm: innate immune sensing of viruses via the unfolded protein response. Front. Microbiol. 5, 222 (2014).
Salazar, M. et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J. Clin. Invest. 119, 1359–1372 (2009).
Bernales, S., McDonald, K. L. & Walter, P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLOS Biol. 4, e423 (2006).
Ogata, M. et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol. Cell. Biol. 26, 9220–9231 (2006).
Yorimitsu, T., Nair, U., Yang, Z. & Klionsky, D. J. Endoplasmic reticulum stress triggers autophagy. J. Biol. Chem. 281, 30299–30304 (2006).
Kedersha, N. L., Gupta, M., Li, W., Miller, I. & Anderson, P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J. Cell Biol. 147, 1431–1442 (1999).
Sessions, O. M. et al. Discovery of insect and human dengue virus host factors. Nature 458, 1047–1050 (2009).
Chan, S. W. Unfolded protein response in hepatitis C virus infection. Front. Microbiol. 5, 233 (2014).
Perera, N., Miller, J. L. & Zitzmann, N. The role of the unfolded protein response in dengue virus pathogenesis. Cell. Microbiol. 19, e12734 (2017).
Pena, J. & Harris, E. Dengue virus modulates the unfolded protein response in a time-dependent manner. J. Biol. Chem. 286, 14226–14236 (2011).
Roth, H. et al. Flavivirus infection uncouples translation suppression from cellular stress responses. mBio 8, e02150-16 (2017).
Ruggieri, A. et al. Dynamic oscillation of translation and stress granule formation mark the cellular response to virus infection. Cell Host Microbe 12, 71–85 (2012). This is an elegant study that reveals the dynamics of stress granule assembly and disassembly and the role of this dynamic in counteracting RNA translation repression and prolonging cell survival.
Edgil, D., Polacek, C. & Harris, E. Dengue virus utilizes a novel strategy for translation initiation when cap-dependent translation is inhibited. J. Virol. 80, 2976–2986 (2006).
Khawaja, A., Vopalensky, V. & Pospisek, M. Understanding the potential of hepatitis C virus internal ribosome entry site domains to modulate translation initiation via their structure and function. Wiley Interdiscip. Rev. RNA 6, 211–224 (2015).
Walsh, D. & Mohr, I. Viral subversion of the host protein synthesis machinery. Nat. Rev. Microbiol. 9, 860–875 (2011).
Brewer, J. W. & Jackowski, S. UPR-mediated membrane biogenesis in B cells. Biochem. Res. Int. 2012, 738471 (2012).
Yoshida, H. et al. A time-dependent phase shift in the mammalian unfolded protein response. Dev. Cell 4, 265–271 (2003).
Ke, P. Y. & Chen, S. S. Activation of the unfolded protein response and autophagy after hepatitis C virus infection suppresses innate antiviral immunity in vitro. J. Clin. Invest. 121, 37–56 (2011).
Wang, J. et al. Hepatitis C virus core protein activates autophagy through EIF2AK3 and ATF6 UPR pathway-mediated MAP1LC3B and ATG12 expression. Autophagy 10, 766–784 (2014).
Yoshida, H., Matsui, T., Yamamoto, A., Okada, T. & Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107, 881–891 (2001).
Viktorovskaya, O. V., Greco, T. M., Cristea, I. M. & Thompson, S. R. Identification of RNA binding proteins associated with dengue virus RNA in infected cells reveals temporally distinct host factor requirements. PLOS Negl. Trop. Dis. 10, e0004921 (2016).
Fink, J. et al. Host gene expression profiling of dengue virus infection in cell lines and patients. PLOS Negl. Trop. Dis. 1, e86 (2007).
Choy, M. M., Sessions, O. M., Gubler, D. J. & Ooi, E. E. Production of infectious dengue virus in Aedes aegypti is dependent on the ubiquitin proteasome pathway. PLOS Negl. Trop. Dis. 9, e0004227 (2015).
Xin, Q. L. et al. Quantitative proteomic analysis of mosquito C6/36 cells reveals host proteins involved in Zika virus infection. J. Virol. 91, e00554-17 (2017).
Choy, M. M. et al. Proteasome inhibition suppresses dengue virus egress in antibody dependent infection. PLOS Negl. Trop. Dis. 9, e0004058 (2015).
Fernandez-Garcia, M. D. et al. Appraising the roles of CBLL1 and the ubiquitin/proteasome system for flavivirus entry and replication. J. Virol. 85, 2980–2989 (2011).
Byk, L. A. et al. Dengue virus genome uncoating requires ubiquitination. mBio 7, e00804-16 (2016).
Poenisch, M. et al. Identification of HNRNPK as regulator of hepatitis C virus particle production. PLOS Pathog. 11, e1004573 (2015).
Li, Q. et al. A genome-wide genetic screen for host factors required for hepatitis C virus propagation. Proc. Natl Acad. Sci. USA 106, 16410–16415 (2009).
Gao, L. et al. Interaction with a ubiquitin-like protein enhances the ubiquitination and degradation of hepatitis C virus RNA-dependent RNA polymerase. J. Virol. 77, 4149–4159 (2003).
Lin, W. et al. Hepatitis C virus expression suppresses interferon signaling by degrading STAT1. Gastroenterology 128, 1034–1041 (2005).
Lin, W. et al. Hepatitis C virus core protein blocks interferon signaling by interaction with the STAT1 SH2 domain. J. Virol. 80, 9226–9235 (2006).
Shao, R. X. et al. Suppressor of cytokine signaling 3 suppresses hepatitis C virus replication in an mTOR-dependent manner. J. Virol. 84, 6060–6069 (2010).
Zhang, X. et al. GP73 represses host innate immune response to promote virus replication by facilitating MAVS and TRAF6 degradation. PLOS Pathog. 13, e1006321 (2017).
Stevenson, N. J. et al. Hepatitis C virus targets the interferon-alpha JAK/STAT pathway by promoting proteasomal degradation in immune cells and hepatocytes. FEBS Lett. 587, 1571–1578 (2013).
Grant, A. et al. Zika virus targets human STAT2 to inhibit type I interferon signaling. Cell Host Microbe 19, 882–890 (2016).
Kumar, A. et al. Zika virus inhibits type-I interferon production and downstream signaling. EMBO Rep. 17, 1766–1775 (2016).
Ashour, J., Laurent-Rolle, M., Shi, P. Y. & Garcia-Sastre, A. NS5 of dengue virus mediates STAT2 binding and degradation. J. Virol. 83, 5408–5418 (2009).
Metz, P. et al. Dengue virus inhibition of autophagic flux and dependency of viral replication on proteasomal degradation of the autophagy receptor p62. J. Virol. 89, 8026–8041 (2015).
Wang, L., Tian, Y. & Ou, J. H. HCV induces the expression of Rubicon and UVRAG to temporally regulate the maturation of autophagosomes and viral replication. PLOS Pathog. 11, e1004764 (2015).
McLean, J. E., Wudzinska, A., Datan, E., Quaglino, D. & Zakeri, Z. Flavivirus NS4A-induced autophagy protects cells against death and enhances virus replication. J. Biol. Chem. 286, 22147–22159 (2011).
Datan, E. et al. Dengue-induced autophagy, virus replication and protection from cell death require ER stress (PERK) pathway activation. Cell Death Dis. 7, e2127 (2016).
Liang, Q. et al. Zika virus NS4A and NS4B proteins deregulate Akt-mTOR signaling in human fetal neural stem cells to inhibit neurogenesis and induce autophagy. Cell Stem Cell 19, 663–671 (2016).
Wu, Y. W. et al. Autophagy-associated dengue vesicles promote viral transmission avoiding antibody neutralization. Sci. Rep. 6, 32243 (2016).
Panyasrivanit, M., Khakpoor, A., Wikan, N. & Smith, D. R. Co-localization of constituents of the dengue virus translation and replication machinery with amphisomes. J. Gen. Virol. 90, 448–456 (2009).
Sir, D. et al. Replication of hepatitis C virus RNA on autophagosomal membranes. J. Biol. Chem. 287, 18036–18043 (2012).
Fahmy, A. M. & Labonte, P. The autophagy elongation complex (ATG5-12/16L1) positively regulates HCV replication and is required for wild-type membranous web formation. Sci. Rep. 7, 40351 (2017).
Guevin, C. et al. Autophagy protein ATG5 interacts transiently with the hepatitis C virus RNA polymerase (NS5B) early during infection. Virology 405, 1–7 (2010).
Dreux, M., Gastaminza, P., Wieland, S. F. & Chisari, F. V. The autophagy machinery is required to initiate hepatitis C virus replication. Proc. Natl Acad. Sci. USA 106, 14046–14051 (2009).
Dreux, M. & Chisari, F. V. Autophagy proteins promote hepatitis C virus replication. Autophagy 5, 1224–1225 (2009).
Shrivastava, S. et al. Knockdown of autophagy inhibits infectious hepatitis C virus release by the exosomal pathway. J. Virol. 90, 1387–1396 (2015).
Tanida, I. et al. Knockdown of autophagy-related gene decreases the production of infectious hepatitis C virus particles. Autophagy 5, 937–945 (2009).
Lennemann, N. J. & Coyne, C. B. Dengue and Zika viruses subvert reticulophagy by NS2B3-mediated cleavage of FAM134B. Autophagy 13, 322–332 (2017).
Heaton, N. S. & Randall, G. Dengue virus-induced autophagy regulates lipid metabolism. Cell Host Microbe 8, 422–432 (2010).
Routhu, N. K. & Byrareddy, S. N. Host-virus interaction of ZIKA virus in modulating disease pathogenesis. J. Neuroimmune Pharmacol. 12, 219–232 (2017).
Jordan, T. X. & Randall, G. Dengue virus activates the AMP kinase-mTOR axis to stimulate a proviral lipophagy. J. Virol. 91, e02020-16 (2017).
Kim, S. J. et al. Hepatitis C virus triggers mitochondrial fission and attenuates apoptosis to promote viral persistence. Proc. Natl Acad. Sci. USA 111, 6413–6418 (2014).
Paul, D., Madan, V. & Bartenschlager, R. Hepatitis C virus RNA replication and assembly: living on the fat of the land. Cell Host. Microbe 16, 569–579 (2014).
Martin-Acebes, M. A., Vazquez-Calvo, A. & Saiz, J. C. Lipids and flaviviruses, present and future perspectives for the control of dengue, Zika, and West Nile viruses. Prog. Lipid Res. 64, 123–137 (2016).
Merino-Ramos, T. et al. Modification of the host cell lipid metabolism induced by hypolipidemic drugs targeting the acetyl coenzyme A carboxylase impairs West Nile virus replication. Antimicrob. Agents Chemother. 60, 307–315 (2015).
Soto-Acosta, R., Bautista-Carbajal, P., Cervantes-Salazar, M., Angel-Ambrocio, A. H. & Del Angel, R. M. DENV up-regulates the HMG-CoA reductase activity through the impairment of AMPK phosphorylation: a potential antiviral target. PLOS Pathog. 13, e1006257 (2017).
Li, Q., Pene, V., Krishnamurthy, S., Cha, H. & Liang, T. J. Hepatitis C virus infection activates an innate pathway involving IKK-alpha in lipogenesis and viral assembly. Nat. Med. 19, 722–729 (2013). This study reveals a novel function of the innate immunity regulator IKK α in the regulation of lipogenic genes. This mechanism is exploited by HCV to promote lipid droplet formation and the assembly of infectious virus particles.
Perera, R. et al. Dengue virus infection perturbs lipid homeostasis in infected mosquito cells. PLOS Pathog. 8, e1002584 (2012).
Martin-Acebes, M. A. et al. The composition of West Nile virus lipid envelope unveils a role of sphingolipid metabolism in flavivirus biogenesis. J. Virol. 88, 12041–12054 (2014).
Diamond, D. L. et al. Temporal proteome and lipidome profiles reveal hepatitis C virus-associated reprogramming of hepatocellular metabolism and bioenergetics. PLOS Pathog. 6, e1000719 (2010).
Zhang, J. et al. Positive-strand RNA viruses stimulate host phosphatidylcholine synthesis at viral replication sites. Proc. Natl Acad. Sci. USA 113, E1064–E1073 (2016).
Wang, H. et al. Oxysterol-binding protein is a phosphatidylinositol 4-kinase effector required for HCV replication membrane integrity and cholesterol trafficking. Gastroenterology 146, 1373–1385.e11 (2014). This study demonstrates that oxysterol-binding protein is recruited to the HCV membranous web in a phosphatidylinositol 4-kinase (PI4K)-dependent manner that is required to regulate cholesterol trafficking to HCV ROs.
Stoeck, I. K. et al. Hepatitis C virus replication depends on endosomal cholesterol homeostasis. J. Virol. 92, e01196–17 (2017).
Harak, C. et al. Mapping of functional domains of the lipid kinase phosphatidylinositol 4-kinase type III alpha involved in enzymatic activity and hepatitis C virus replication. J. Virol. 88, 9909–9926 (2014).
Khan, I. et al. Modulation of hepatitis C virus genome replication by glycosphingolipids and four-phosphate adaptor protein 2. J. Virol. 88, 12276–12295 (2014).
Zhang, J. et al. Small G Rac1 is involved in replication cycle of dengue serotype 2 virus in EAhy926 cells via the regulation of actin cytoskeleton. Sci. China Life Sci. 59, 487–494 (2016).
Zamudio-Meza, H., Castillo-Alvarez, A., Gonzalez-Bonilla, C. & Meza, I. Cross-talk between Rac1 and Cdc42 GTPases regulates formation of filopodia required for dengue virus type-2 entry into HMEC-1 cells. J. Gen. Virol. 90, 2902–2911 (2009).
Teo, C. S. & Chu, J. J. Cellular vimentin regulates construction of dengue virus replication complexes through interaction with NS4A protein. J. Virol. 88, 1897–1913 (2014).
Chen, W. et al. Vimentin is required for dengue virus serotype 2 infection but microtubules are not necessary for this process. Arch. Virol. 153, 1777–1781 (2008).
Roohvand, F. et al. Initiation of hepatitis C virus infection requires the dynamic microtubule network: role of the viral nucleocapsid protein. J. Biol. Chem. 284, 13778–13791 (2009).
Bost, A. G., Venable, D., Liu, L. & Heinz, B. A. Cytoskeletal requirements for hepatitis C virus (HCV) RNA synthesis in the HCV replicon cell culture system. J. Virol. 77, 4401–4408 (2003).
Akil, A. et al. Septin 9 induces lipid droplets growth by a phosphatidylinositol-5-phosphate and microtubule-dependent mechanism hijacked by HCV. Nat. Commun. 7, 12203 (2016). This study reports that septin 9 can modulate the growth of lipid droplets through microtubule-dependent and PtdIns5P-dependent mechanisms. Such an ability is exploited by HCV to create an environment favourable for its replication.
Mettenleiter, T. C. Breaching the barrier-the nuclear envelope in virus infection. J. Mol. Biol. 428, 1949–1961 (2016).
Byk, L. A. & Gamarnik, A. V. Properties and functions of the dengue virus capsid protein. Annu. Rev. Virol. 3, 263–281 (2016).
Kumar, A. et al. Nuclear localization of dengue virus nonstructural protein 5 does not strictly correlate with efficient viral RNA replication and inhibition of type I interferon signaling. J. Virol. 87, 4545–4557 (2013).
Hannemann, H. et al. Serotype-specific differences in dengue virus non-structural protein 5 nuclear localization. J. Biol. Chem. 288, 22621–22635 (2013).
Bonamassa, B. et al. Hepatitis C virus and host cell nuclear transport machinery: a clandestine affair. Front. Microbiol. 6, 619 (2015).
Levin, A. et al. Functional characterization of nuclear localization and export signals in hepatitis C virus proteins and their role in the membranous web. PLOS ONE 9, e114629 (2014).
Overby, A. K. & Weber, F. Hiding from intracellular pattern recognition receptors, a passive strategy of flavivirus immune evasion. Virulence 2, 238–240 (2011).
Lussignol, M. et al. Proteomics of HCV virions reveals an essential role for the nucleoporin Nup98 in virus morphogenesis. Proc. Natl Acad. Sci. USA 113, 2484–2489 (2016). This study determines the protein composition of HCV virions and identifies multiple cellular proteins, with nuclear pore complex protein NUP98 playing an important role in virus propagation.
Ye, J. et al. Japanese encephalitis virus NS5 inhibits type I interferon (IFN) production by blocking the nuclear translocation of IFN regulatory factor 3 and NF-kappaB. J. Virol. 91, e00039-17 (2017).
Gagne, B., Tremblay, N., Park, A. Y., Baril, M. & Lamarre, D. Importin beta1 targeting by hepatitis C virus NS3/4A protein restricts IRF3 and NF-kappaB signaling of IFNB1 antiviral response. Traffic 18, 362–377 (2017).
Miyanari, Y. et al. The lipid droplet is an important organelle for hepatitis C virus production. Nat. Cell Biol. 9, 1089–1097 (2007).
Vance, J. E. MAM (mitochondria-associated membranes) in mammalian cells: lipids and beyond. Biochim. Biophys. Acta 1841, 595–609 (2014).
Barbier, V., Lang, D., Valois, S., Rothman, A. L. & Medin, C. L. Dengue virus induces mitochondrial elongation through impairment of Drp1-triggered mitochondrial fission. Virology 500, 149–160 (2017).
Yu, C. Y. et al. Dengue virus impairs mitochondrial fusion by cleaving mitofusins. PLOS Pathog. 11, e1005350 (2015).
Aguirre, S. et al. Dengue virus NS2B protein targets cGAS for degradation and prevents mitochondrial DNA sensing during infection. Nat. Microbiol. 2, 17037 (2017). This is a study demonstrating a block of type I interferon activation through NS2B-mediated lysosomal degradation of the cytosolic DNA sensor cyclic GMP–AMP synthase (cGAS), preventing its activation by DENV-induced mitochondrial DNA release.
Siu, G. K. et al. Hepatitis C virus NS5A protein cooperates with phosphatidylinositol 4-kinase IIIalpha to induce mitochondrial fragmentation. Sci. Rep. 6, 23464 (2016).
Ruggieri, V. et al. Hepatitis C virus, mitochondria and auto/mitophagy: exploiting a host defense mechanism. World J. Gastroenterol. 20, 2624–2633 (2014).
Paul, D. & Bartenschlager, R. Architecture and biogenesis of plus-strand RNA virus replication factories. World J. Virol. 2, 32–48 (2013). This review describes the different architectures of positive-strand RNA virus replication factories and discusses the mechanisms of their biogenesis and maintenance.
Le Sommer, C., Barrows, N. J., Bradrick, S. S., Pearson, J. L. & Garcia-Blanco, M. A. G protein-coupled receptor kinase 2 promotes flaviviridae entry and replication. PLOS Negl. Trop. Dis. 6, e1820 (2012).
Krishnan, M. N. et al. RNA interference screen for human genes associated with West Nile virus infection. Nature 455, 242–245 (2008).
Modis, Y., Ogata, S., Clements, D. & Harrison, S. C. Structure of the dengue virus envelope protein after membrane fusion. Nature 427, 313–319 (2004). This is an elegant study describing the three-dimensional structure of the soluble ectodomain of the dengue virus envelope protein in its trimeric, that is, post-fusion state. The structure was used to propose a model for membrane fusion by class II viral fusion proteins.
Zhang, X. et al. Structure of acidic pH dengue virus showing the fusogenic glycoprotein trimers. J. Virol. 89, 743–750 (2015).
Scaturro, P., Cortese, M., Chatel-Chaix, L., Fischl, W. & Bartenschlager, R. Dengue virus non-structural protein 1 modulates infectious particle production via interaction with the structural proteins. PLOS Pathog. 11, e1005277 (2015).
Yu, I. M. et al. Structure of the immature dengue virus at low pH primes proteolytic maturation. Science 319, 1834–1837 (2008). This is an elegant study showing that immature DENV particles undergo a reversible conformational change at acidic pH, rendering them accessible for furin cleavage. Upon cleavage in the trans -Golgi network, the pr cleavage product is retained on the virion to prevent premature membrane fusion.
Zayas, M., Long, G., Madan, V. & Bartenschlager, R. Coordination of hepatitis C virus assembly by distinct regulatory regions in nonstructural protein 5A. PLOS Pathog. 12, e1005376 (2016).
Lee, J. Y. et al. Apolipoprotein E likely contributes to a maturation step of infectious hepatitis C virus particles and interacts with viral envelope glycoproteins. J. Virol. 88, 12422–12437 (2014).
Fukuhara, T. et al. Host-derived apolipoproteins play comparable roles with viral secretory proteins Erns and NS1 in the infectious particle formation of Flaviviridae. PLOS Pathog. 13, e1006475 (2017).
Piver, E. et al. Ultrastructural organisation of HCV from the bloodstream of infected patients revealed by electron microscopy after specific immunocapture. Gut 66, 1487–1495 (2016).
Yang, Z. et al. Neglected but important role of apolipoprotein E exchange in hepatitis C virus infection. J. Virol. 90, 9632–9643 (2016).
Bankwitz, D. et al. Maturation of secreted HCV particles by incorporation of secreted ApoE protects from antibodies by enhancing infectivity. J. Hepatol. 67, 480–489 (2017).
Acosta, E. G. & Bartenschlager, R. The quest for host targets to combat dengue virus infections. Curr. Opin. Virol. 20, 47–54 (2016).
Zeisel, M. B., Crouchet, E., Baumert, T. F. & Schuster, C. Host-targeting agents to prevent and cure hepatitis C virus infection. Viruses 7, 5659–5685 (2015).
Pawlotsky, J. M. et al. Alisporivir plus ribavirin, interferon free or in combination with pegylated interferon, for hepatitis C virus genotype 2 or 3 infection. Hepatology 62, 1013–1023 (2015).
Zeuzem, S. et al. Randomised clinical trial: alisporivir combined with peginterferon and ribavirin in treatment-naive patients with chronic HCV genotype 1 infection (ESSENTIAL II). Aliment. Pharmacol. Ther. 42, 829–844 (2015).
Low, J. G. et al. Efficacy and safety of celgosivir in patients with dengue fever (CELADEN): a phase 1b, randomised, double-blind, placebo-controlled, proof-of- concept trial. Lancet Infect. Dis. 14, 706–715 (2014).
Sung, C. et al. Extended evaluation of virological, immunological and pharmacokinetic endpoints of CELADEN: a randomized, placebo-controlled trial of celgosivir in dengue fever patients. PLOS Negl. Trop. Dis. 10, e0004851 (2016).
Watanabe, S. et al. Optimizing celgosivir therapy in mouse models of dengue virus infection of serotypes 1 and 2: the search for a window for potential therapeutic efficacy. Antiviral Res. 127, 10–19 (2016).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02569827 (2017).
Acknowledgements
The authors apologize to all colleagues whose work they could not cite due to space limitations. Research in the authors' laboratory is supported by the Deutsche Forschungsgemeinschaft (SFB 1129, TP 11; SFB/TRR 83, TP 13; SFB/TRR179, TP 9), the Federal Ministry for Education and Research (BMBF) 'ERA-Net for Applied Systems Biology' (ERASysAPP) (SysVirDrug, grant no. 031A602B) and the EU H2020 project 'ANTIVIRALS' (grant no. 642434), all to R.B. C.J.N. is supported by the European Molecular Biology Organization (EMBO) (ALTF 466–2016).
Author information
Authors and Affiliations
Contributions
C.J.N., M.C. and E.G.A. researched data for the article. C.J.N., M.C., E.G.A. and R.B. substantially contributed to discussion of content, wrote the article and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Guillain–Barré syndrome
-
An acute neurological disease (usually reversible) resulting from autoimmune destruction of the peripheral nervous system that is frequently triggered by infection.
- Microcephaly
-
A neurological condition of abnormal brain development that causes substantially smaller infant head circumferences relative to age-matched controls.
- Tropism
-
Tissue specificity of a virus. Tropism is determined primarily by the presence of membrane receptors that can be exploited by the virus to gain access to host cells.
- Internal ribosome entry site
-
(IRES). A folded RNA element capable of recruiting the small ribosomal subunit that also mediates cap-independent initiation of RNA translation. IRES elements require only a subset of the canonical translation initiation factors, which are determined by the specific type of IRES.
- Polyprotein
-
A polypeptide composed of individual domains that are released both co-translationally and post-translationally by proteolytic cleavage to produce functionally distinct proteins.
- Very-low-density lipoprotein
-
(VLDL). A liver-produced plasma lipoprotein particle ∼30–70 nm in diameter involved in cholesterol and triglyceride transport. Low-density lipoprotein and VLDL contain distinct sets of apolipoproteins.
- Cyclophilin
-
Peptidyl-prolyl isomerases (PPIases) that catalyse the cis–trans isomerization of peptide bonds at proline residues and facilitate protein folding.
- Oligosaccharyltransferase
-
(OST). A heteromeric transmembrane protein complex located in the endoplasmic reticulum lumen that catalyses the transfer of a pre-assembled oligosaccharide to selected asparagine residues within the consensus sequence Asn-X-Ser/Thr of nascent polypeptides.
- Ubiquitin-dependent proteasome system
-
(UPS). A multicomponent system for regulated protein degradation. Proteins are marked for degradation by conjugation with the ubiquitin polypeptide through the concerted action of modular conjugation machinery. Ubiquitylated substrates are recognized, unfolded and degraded by a large multisubunit protease complex called the proteasome.
- CRISPR–Cas9
-
A site-specific gene-editing system derived from a bacterial adaptive defence system that retains foreign DNA in the CRISPR gene locus. The system is composed of a guide RNA homologous to the target gene and the CRISPR–Cas9 nuclease. Sequence complementarity guides Cas9 to the specific region targeted for cleavage.
- Bortezomib
-
A dipeptide boronic acid derivate that reversibly inhibits the chymotryptic activity of the proteasome. It was the first therapeutic proteasome inhibitor approved by the FDA for clinical use as an anticancer drug.
- Restriction factors
-
Cellular factors that inhibit pathogen replication.
- AKT1–mTOR
-
An intracellular signalling pathway that regulates several cellular processes, including cell proliferation and survival.
- Exosomes
-
Small extracellular vesicles released directly from cells or upon fusion of multivesicular bodies with the plasma membrane.
- Lipophagy
-
An autophagic pathway that selectively targets lipid droplets and mobilizes lipids to be used for energy production.
- ER-phagy
-
An autophagic pathway that selectively sequesters and degrades portions of the endoplasmic reticulum.
- Parkin
-
An E3 ubiquitin ligase that is involved in autophagic elimination of damaged mitochondria (mitophagy). Mutations in the parkin gene are linked to autosomal recessive juvenile Parkinson disease.
- Sterol regulatory element-binding protein
-
(SREBP). A membrane-bound transcription factor that regulates the transcription of genes involved in fatty acids and cholesterol metabolism.
- Microfilament
-
Also known as actin filaments; a component of the cytoskeleton. Microfilaments range from 7–9 nm in diameter and are composed of actin polymers. Microfilaments are involved in several cellular processes, including cytokinesis, cell movement, endocytosis and muscle contraction.
- Septins
-
GTPases that assemble into repeated hetero-oligomers and polymerize into higher-order structures, such as rings or filaments. Septins are the fourth cytoskeletal component and take part in several cellular processes, such as cell division, migration and pathogen interactions.
- Interferon regulatory factor 3
-
(IRF3). A transcription factor that mediates the expression of type 1 interferons and interferon-stimulated genes.
- Dynamin 1-like protein
-
(DRP1). A GTPase of the dynamin superfamily that mediate mitochondrial outer membrane fission events.
Rights and permissions
About this article
Cite this article
Neufeldt, C., Cortese, M., Acosta, E. et al. Rewiring cellular networks by members of the Flaviviridae family. Nat Rev Microbiol 16, 125–142 (2018). https://doi.org/10.1038/nrmicro.2017.170
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro.2017.170
This article is cited by
-
From viruses to cancer: exploring the role of the hepatitis C virus NS3 protein in carcinogenesis
Infectious Agents and Cancer (2024)
-
The endoplasmic reticulum (ER): a crucial cellular hub in flavivirus infection and potential target site for antiviral interventions
npj Viruses (2024)
-
Glycerophospholipid remodeling is critical for orthoflavivirus infection
Nature Communications (2024)
-
Interaction of chikungunya virus glycoproteins with macrophage factors controls virion production
The EMBO Journal (2024)
-
Inhibition of sterol O-acyltransferase 1 blocks Zika virus infection in cell lines and cerebral organoids
Communications Biology (2024)