Key Points
-
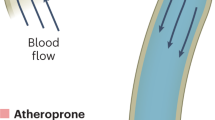
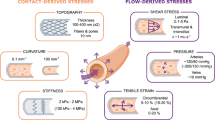
Fluid shear stress from blood flow and circumferential stretch of vessel walls from blood pressure are important regulatory factors that control the morphogenesis and physiology of blood vessels. However, atherosclerosis initiates at regions of arteries that, owing to vessel anatomy, develop disturbances in flow patterns.
-
Mechanical forces from blood flow are exerted on many different structural elements in the cell. A number of these might transduce forces into biochemical signals that regulate cellular responses.
-
Disturbed fluid shear stress activates the vascular endothelium to initiate atherosclerosis, mainly because cells cannot adapt to these flow patterns and cannot downregulate signalling pathways. Changes in gene expression and the endothelial extracellular matrix help entrain the activated state to cause life-long chronic inflammation.
-
Systemic risk factors, such as high cholesterol and blood pressure, synergize with disturbed flow to promote the progression of atherosclerosis.
-
These ideas suggest that atherosclerosis arises because the normal physiological responses to laminar flow have unintended consequences in the face of disturbed flow.
Abstract
Forces that are associated with blood flow are major determinants of vascular morphogenesis and physiology. Blood flow is crucial for blood vessel development during embryogenesis and for regulation of vessel diameter in adult life. It is also a key factor in atherosclerosis, which, despite the systemic nature of major risk factors, occurs mainly in regions of arteries that experience disturbances in fluid flow. Recent data have highlighted the potential endothelial mechanotransducers that might mediate responses to blood flow, the effects of atheroprotective rather than atherogenic flow, the mechanisms that contribute to the progression of the disease and how systemic factors interact with flow patterns to cause atherosclerosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hynes, R. O. & Zhao, Q. The evolution of cell adhesion. J. Cell Biol. 150, F89–F96 (2000).
Hove, J. R. et al. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 421, 172–177 (2003).
Lucitti, J. L. et al. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development 134, 3317–3326 (2007). Provides elegant evidence that fluid shear stress mediates the rearrangement of a primitive vascular plexus into a mature vascular tree in early mouse embryos.
Lusis, A. J. Atherosclerosis. Nature 407, 233–241 (2000). A good review that discusses the initiation and progression of atherosclerosis with emphasis on genetics, lipids and cellular interactions.
Birukov, K. G. et al. Intraluminal pressure is essential for the maintenance of smooth muscle caldesmon and filamin content in aortic organ culture. Arterioscler. Thromb. Vasc. Biol. 18, 922–927 (1998).
Martinez-Lemus, L. A. et al. Integrins as unique receptors for vascular control. J. Vasc. Res. 40, 211–233 (2003).
Folkow, B. Early structural changes in hypertension: pathophysiology and clinical consequences. J. Cardiovasc. Pharmacol. 22, S1–S6 (1993).
Lehoux, S., Castier, Y. & Tedgui, A. Molecular mechanisms of the vascular responses to haemodynamic forces. J. Intern. Med. 259, 381–392 (2006).
Campbell, W. B. & Falck, J. R. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension 49, 590–596 (2007).
Vanhoutte, P. M., Boulanger, C. M. & Mombouli, J. V. Endothelium-derived relaxing factors and converting enzyme inhibition. Am. J. Cardiol. 76, 3E–12E (1995).
Haddy, F. J., Vanhoutte, P. M. & Feletou, M. Role of potassium in regulating blood flow and blood pressure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R546–R552 (2006).
Di Stefano, I., Koopmans, D. R. & Langille, B. L. Modulation of arterial growth of the rabbit carotid artery associated with experimental elevation of blood flow. J. Vasc. Res. 35, 1–7 (1998).
Brownlee, R. D. & Langille, B. L. Arterial adaptations to altered blood flow. Can. J. Physiol. Pharmacol. 69, 978–983 (1991).
Baffert, F. et al. Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am. J. Physiol. Heart Circ. Physiol. 290, H547–H559 (2006).
Meeson, A., Palmer, M., Calfon, M. & Lang, R. A relationship between apoptosis and flow during programmed capillary regression is revealed by vital analysis. Development 122, 3929–3938 (1996).
Li, Y. S., Haga, J. H. & Chien, S. Molecular basis of the effects of shear stress on vascular endothelial cells. J. Biomech. 38, 1949–1971 (2005).
Orr, A. W., Helmke, B. P., Blackman, B. R. & Schwartz, M. A. Mechanisms of mechanotransduction. Dev. Cell 10, 11–20 (2006).
Davies, P. F. Flow-mediated endothelial mechanotransduction. Physiol. Rev. 75, 519–560 (1995).
Helmke, B. P., Thakker, D. B., Goldman, R. D. & Davies, P. F. Spatiotemporal analysis of flow-induced intermediate filament displacement in living endothelial cells. Biophys. J. 80, 184–194 (2001).
Schiffers, P. M. et al. Altered flow-induced arterial remodeling in vimentin-deficient mice. Arterioscler. Thromb. Vasc. Biol. 20, 611–616 (2000).
Hutcheson, I. R. & Griffith, T. M. Mechanotransduction through the endothelial cytoskeleton: mediation of flow- but not agonist-induced EDRF release. Br. J. Pharmacol. 118, 720–726 (1996).
Malek, A. M., Zhang, J., Jiang, J., Alper, S. L. & Izumo, S. Endothelin-1 gene suppression by shear stress: pharmacological evaluation of the role of tyrosine kinase, intracellular calcium, cytoskeleton, and mechanosensitive channels. J. Mol. Cell. Cardiol. 31, 387–399 (1999).
Tzima, E. et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437, 426–431 (2005). Identifies a complex that consists of PECAM1, VE-cadherin and VEGFR2 in the pathway leading to integrin activation and induction of NF-κB by flow.
Jin, Z. G. et al. Ligand independent activation of VEGF receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthesis. Circ. Res. 93, 354–363 (2003).
Shay-Salit, A. et al. VEGF receptor 2 and the adherens junction as a mechanical transducer in vascular endothelial cells. Proc. Natl Acad. Sci. USA 99, 9462–9467 (2002).
Jin, Z. G., Wong, C., Wu, J. & Berk, B. C. Flow shear stress stimulates Gab1 tyrosine phosphorylation to mediate protein kinase B and endothelial nitric-oxide synthase activation in endothelial cells. J. Biol. Chem. 280, 12305–12309 (2005).
Tzima, E. et al. Identification of a mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437, 426–431 (2005).
Fleming, I., Fisslthaler, B., Dixit, M. & Busse, R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J. Cell Sci. 118, 4103–4111 (2005).
Tzima, E., del Pozo, M. A., Shattil, S. S., Chien, S. & Schwartz, M. A. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 20, 4639–4647 (2001).
Tzima, E. et al. Activation of Rac in endothelial cells in response to fluid shear stress mediates gene expression and cell alignment. EMBO J. 21, 6791–6800 (2002).
Tzima, E., Kiosses, W. B., del Pozo, M. A. & Schwartz, M. A. Localized Cdc42 activation detected using a novel assay mediates MTOC positioning in endothelial cells in response to fluid shear stress. J. Biol. Chem. 278, 31020–31023 (2003).
Tzima, E. et al. Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. EMBO J. 21, 6791–6800 (2002).
Bhullar, I. S. et al. Fluid shear stress activation of IκB kinase is integrin-dependent. J. Biol. Chem. 273, 30544–30549 (1998).
Chen, K. D. et al. Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases, integrins, and Shc. J. Biol. Chem. 274, 18393–18400 (1999).
Muller, J. M., Chilian, W. M. & Davis, M. J. Integrin signaling transduces shear stress-dependent vasodilation of coronary arterioles. Circ. Res. 80, 320–326 (1997).
Ishida, T., Peterson, T. E., Kovach, N. L. & Berk, B. C. MAP kinase activation by flow in endothelial cells. Role of β1 integrins and tyrosine kinases. Circ. Res. 79, 310–316 (1996).
Katsumi, A., Orr, A. W., Tzima, E. & Schwartz, M. A. Integrins in mechanotransduction. J. Biol. Chem. 279, 12001–12004 (2004).
Butler, P. J., Norwich, G., Weinbaum, S. & Chien, S. Shear stress induces a time- and position-dependent increase in endothelial cell membrane fluidity. Am. J. Physiol. Cell Physiol. 280, C962–C969 (2001).
Gudi, S., Nolan, J. P. & Frangos, J. A. Modulation of GTPase activity of G proteins by fluid shear stress and phospholipid composition. Proc. Natl Acad. Sci. USA 95, 2515–2519 (1998).
White, C. R. & Frangos, J. A. The shear stress of it all: the cell membrane and mechanochemical transduction. Phil. Trans. R. Soc. Lond. B 362, 1459–1467 (2007).
Bergaya, S. et al. Decreased flow-dependent dilation in carotid arteries of tissue kallikrein-knockout mice. Circ. Res. 88, 593–599 (2001).
Maroto, R. et al. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nature Cell Biol. 7, 179–185 (2005).
Hoger, J. H., Ilyin, V. I., Forsyth, S. & Hoger, A. Shear stress regulates the endothelial Kir2.1 ion channel. Proc. Natl Acad. Sci. USA 99, 7780–7785 (2002). Shows that an inward-rectifying K+ channel opens in response to flow and analyzes the mechanism of activation.
Zaritsky, J. J., Eckman, D. M., Wellman, G. C., Nelson, M. T. & Schwarz, T. L. Targeted disruption of Kir2.1 and Kir2.2 genes reveals the essential role of the inwardly rectifying K+ current in K+-mediated vasodilation. Circ. Res. 87, 160–166 (2000).
Fang, Y. et al. Functional expression of Kir2.x in human aortic endothelial cells: the dominant role of Kir2.2. Am. J. Physiol., Cell Physiol. 289, C1134–C1144 (2005).
Bodin, P., Bailey, D. & Burnstock, G. Increased flow-induced ATP release from isolated vascular endothelial cells but not smooth muscle cells. Br. J. Pharmacol. 103, 1203–1205 (1991).
Faigle, M., Seessle, J., Zug, S., El Kasmi, K. C. & Eltzschig, H. K. ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia. PLoS ONE 3, e2801 (2008).
Sabirov, R. Z. & Okada, Y. ATP release via anion channels. Purinergic Signal. 1, 311–328 (2005).
Yamamoto, K. et al. Involvement of cell surface ATP synthase in flow-induced ATP release by vascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 293, H1646–H1653 (2007).
Choi, H. W., Ferrara, K. W. & Barakat, A. I. Modulation of ATP/ADP concentration at the endothelial surface by shear stress: effect of flow recirculation. Ann. Biomed. Eng. 35, 505–516 (2007).
Smith, M. L., Long, D. S., Damiano, E. R. & Ley, K. Near-wall micro-PIV reveals a hydrodynamically relevant endothelial surface layer in venules in vivo. Biophys. J. 85, 637–645 (2003).
Vink, H. & Duling, B. R. Capillary endothelial surface layer selectively reduces plasma solute distribution volume. Am. J. Physiol. Heart Circ. Physiol. 278, H285–H289 (2000).
Weinbaum, S., Tarbell, J. M. & Damiano, E. R. The structure and function of the endothelial glycocalyx layer. Annu. Rev. Biomed. Eng. 9, 121–167 (2007).
Schwartz, M. A. & Assoian, R. K. Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J. Cell Sci. 114, 2553–2560 (2001).
Weimbs, T. Polycystic kidney disease and renal injury repair: common pathways, fluid flow, and the function of polycystin-1. Am. J. Physiol. Renal Physiol. 293, F1423–F1432 (2007).
Kim, K., Drummond, I., Ibraghimov-Beskrovnaya, O., Klinger, K. & Arnaout, M. A. Polycystin 1 is required for the structural integrity of blood vessels. Proc. Natl Acad. Sci. USA 97, 1731–1736 (2000).
Iomini, C., Tejada, K., Mo, W., Vaananen, H. & Piperno, G. Primary cilia of human endothelial cells disassemble under laminar shear stress. J. Cell Biol. 164, 811–817 (2004).
Van der Heiden, K. et al. Endothelial primary cilia in areas of disturbed flow are at the base of atherosclerosis. Atherosclerosis 196, 542–550 (2007).
Wilson, P. D. Polycystin: new aspects of structure, function, and regulation. J. Am. Soc. Nephrol. 12, 834–845 (2001).
Malek, A. M., Alper, S. L. & Izumo, S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 282, 2035–2042 (1999).
Brooks, A. R., Lelkes, P. I. & Rubanyi, G. M. Gene expression profiling of vascular endothelial cells exposed to fluid mechanical forces: relevance for focal susceptibility to atherosclerosis. Endothelium 11, 45–57 (2004).
Chappell, D. C., Varner, S. E., Nerem, R. M., Medford, R. M. & Alexander, R. W. Oscillatory shear stress stimulates adhesion molecule expression in cultured human endothelium. Circ. Res. 82, 532–539 (1998).
Jongstra-Bilen, J. et al. Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J. Exp. Med. 203, 2073–2083 (2006).
Berk, B. C., Abe, J. I., Min, W., Surapisitchat, J. & Yan, C. Endothelial atheroprotective and anti-inflammatory mechanisms. Ann. NY Acad. Sci. 947, 93–109 (2001).
Dekker, R. J. et al. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2). Blood 100, 1689–1698 (2002).
Wang, N. et al. Shear stress regulation of Kruppel-like factor 2 expression is flow pattern-specific. Biochem. Biophys. Res. Commun. 341, 1244–1251 (2006).
Dekker, R. J. et al. KLF2 provokes a gene expression pattern that establishes functional quiescent differentiation of the endothelium. Blood 107, 4354–4363 (2006).
SenBanerjee, S. et al. KLF2 is a novel transcriptional regulator of endothelial proinflammatory activation. J. Exp. Med. 199, 1305–1315 (2004).
Parmar, K. M. et al. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J. Clin. Invest. 116, 49–58 (2006).
Hsieh, H. J. et al. Increase of reactive oxygen species (ROS) in endothelial cells by shear flow and involvement of ROS in shear-induced c-fos expression. J. Cell. Physiol. 175, 156–162 (1998).
Cicha, I., Goppelt-Struebe, M., Yilmaz, A., Daniel, W. G. & Garlichs, C. D. Endothelial dysfunction and monocyte recruitment in cells exposed to non-uniform shear stress. Clin. Hemorheol. Microcirc. 39, 113–119 (2008).
Zhao, S. et al. Synergistic effects of fluid shear stress and cyclic circumferential stretch on vascular endothelial cell morphology and cytoskeleton. Arterioscler. Thromb. Vasc. Biol. 15, 1781–1786 (1995).
Mohan, S., Mohan, N. & Sprague, E. A. Differential activation of NF-kB in human aortic endothelial cells conditioned to specific flow environments. Am. J. Physiol., Cell Physiol. 273, C572–C578 (1997).
Cheng, C. et al. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation 113, 2744–2753 (2006).
Civelekoglu-Scholey, G. et al. Model of coupled transient changes of Rac, Rho, adhesions and stress fibers alignment in endothelial cells responding to shear stress. J. Theor. Biol. 232, 569–585 (2005).
Mowbray, A. L., Kang, D. H., Rhee, S. G., Kang, S. W. & Jo, H. Laminar shear stress up-regulates peroxiredoxins (PRX) in endothelial cells: PRX 1 as a mechanosensitive antioxidant. J. Biol. Chem. 283, 1622–1627 (2008).
Dai, G. et al. Biomechanical forces in atherosclerosis-resistant vascular regions regulate endothelial redox balance via phosphoinositol 3-kinase/Akt-dependent activation of Nrf2. Circ. Res. 101, 723–733 (2007).
Yamawaki, H., Pan, S., Lee, R. T. & Berk, B. C. Fluid shear stress inhibits vascular inflammation by decreasing thioredoxin-interacting protein in endothelial cells. J. Clin. Invest. 115, 733–738 (2005). An important study that reveals a novel mechanism by which laminar shear stress inhibits oxidative stress and inflammatory activation of endothelial cells.
Passerini, A. G. et al. Coexisting proinflammatory and antioxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proc. Natl Acad. Sci. USA 101, 2482–2487 (2004).
Canfield, A. E. et al. The involvement of matrix glycoproteins in vascular calcification and fibrosis: an immunohistochemical study. J. Pathol. 196, 228–234 (2002).
Orr, A. W. et al. The subendothelial extracellular matrix modulates NF-κB activation by flow: a potential role in atherosclerosis. J. Cell Biol. 169, 191–202 (2005).
Shekhonin, B. V., Domogatsky, S. P., Idelson, G. L., Koteliansky, V. E. & Rukosuev, V. S. Relative distribution of fibronectin and type I, III, IV, V collagens in normal and atherosclerotic intima of human arteries. Atherosclerosis 67, 9–16 (1987).
Smith, E. B. Fibrinogen, fibrin and fibrin degradation products in relation to atherosclerosis. Clin. Haematol. 15, 355–370 (1986).
Klekotka, P. A., Santoro, S. A. & Zutter, M. M. α2 integrin subunit cytoplasmic domain-dependent cellular migration requires p38 MAPK. J. Biol. Chem. 276, 9503–9511 (2001).
Klein, S. et al. α5β1 integrin activates an NF-κB-dependent program of gene expression important for angiogenesis and inflammation. Mol. Cell. Biol. 22, 5912–5922 (2002).
Scatena, M. et al. NF-κB mediates αvβ3 integrin-induced endothelial cell survival. J. Cell Biol. 141, 1083–1093 (1998).
Orr, A. W. et al. Matrix-specific p21-activated kinase activation regulates vascular permeability in atherogenesis. J. Cell Biol. 176, 719–727 (2007).
Tan, M. H. et al. Deletion of the alternatively spliced fibronectin EIIIA domain in mice reduces atherosclerosis. Blood 104, 11–18 (2004).
Guan, J. L., Trevithick, J. E. & Hynes, R. O. Retroviral expression of alternatively spliced forms of rat fibronectin. J. Cell Biol. 110, 833–847 (1990).
Thoumine, O., Nerem, R. M. & Girard, P. R. Changes in organization and composition of the extracellular matrix underlying cultured endothelial cells exposed to laminar steady shear stress. Lab. Invest. 73, 565–576 (1995).
Thoumine, O., Nerem, R. M. & Girard, P. R. Oscillatory shear stress and hydrostatic pressure modulate cell–matrix attachment proteins in cultured endothelial cells. In Vitro Cell. Dev. Biol. Anim. 31, 45–54 (1995).
Chen, S., Mukherjee, S., Chakraborty, C. & Chakrabarti, S. High glucose-induced, endothelin-dependent fibronectin synthesis is mediated via NF-κB and AP-1. Am. J. Physiol., Cell Physiol. 284, C263–C272 (2003).
Jankowski, P., Bilo, G. & Kawecka-Jaszcz, K. The pulsatile component of blood pressure: its role in the pathogenesis of atherosclerosis. Blood Press. 16, 238–245 (2007).
Adhikari, N., Charles, N., Lehmann, U. & Hall, J. L. Transcription factor and kinase-mediated signaling in atherosclerosis and vascular injury. Curr. Atheroscler. Rep. 8, 252–260 (2006).
Kaunas, R., Usami, S. & Chien, S. Regulation of stretch-induced JNK activation by stress fiber orientation. Cell Signal. 18, 1924–1931 (2006). Demonstrates a fascinating dependence of JNK activation on the orientation of mechanical stretch relative to the actin stress fibres.
Dancu, M. B. & Tarbell, J. M. Large negative stress phase angle (SPA) attenuates nitric oxide production in bovine aortic endothelial cells. J. Biomech. Eng. 128, 329–334 (2006).
Harrison, D. G. et al. Endothelial mechanotransduction, nitric oxide and vascular inflammation. J. Intern. Med. 259, 351–363 (2006).
Maxfield, F. R. & Tabas, I. Role of cholesterol and lipid organization in disease. Nature 438, 612–621 (2005).
Stocker, R. & Keaney, J. F. Jr. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 84, 1381–1478 (2004).
Blair, S. N. et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA 276, 205–210 (1996).
Britten, M. B., Zeiher, A. M. & Schachinger, V. Clinical importance of coronary endothelial vasodilator dysfunction and therapeutic options. J. Intern. Med. 245, 315–327 (1999).
Gaba, M. K., Gaba, S. & Clark, L. T. Cardiovascular disease in patients with diabetes: clinical considerations. J. Assoc. Acad. Minor. Phys. 10, 15–22 (1999).
Kaur, H. et al. Diabetes-induced extracellular matrix protein expression is mediated by transcription coactivator p300. Diabetes 55, 3104–3111 (2006).
Stephens, J. W., Khanolkar, M. P. & Bain, S. C. The biological relevance and measurement of plasma markers of oxidative stress in diabetes and cardiovascular disease. Atherosclerosis 20 Jun 2008 (doi:10.1016/j.atherosclerosis.2008.06.006).
Rossant, J. & Howard, L. Signaling pathways in vascular development. Annu. Rev. Cell Dev. Biol. 18, 541–573 (2002).
Cunningham, K. S. & Gotlieb, A. I. The role of shear stress in the pathogenesis of atherosclerosis. Lab. Invest. 85, 9–23 (2005).
Geiger, B., Spatz, J. P. & Bershadsky, A. D. Environmental sensing by cells through focal adhesions. Nature Rev. Mol. Cell Biol. 23 Dec 2008 (doi:10.1038/nrm2593)
Jaalouk, D. E. & Lammerding, J. Mechanotransduction gone awry. Nature Rev. Mol. Cell Biol. 23 Dec 2008 (doi:10.1038/nrm2597).
Acknowledgements
Work from the laboratory of M.S. was supported by National Institutes of Health grants RO1 HL75092 and 80956 to MAS.
Author information
Authors and Affiliations
Corresponding author
Related links
Glossary
- Shear stress
-
The frictional force per unit area that a fluid exerts as it flows over a surface. This force is parallel to the surface and is proportional to the viscosity and the velocity of the fluid, and is inversely proportional to the radius of the vessel.
- Blood pressure
-
The hydraulic pressure (force per area) in the blood vessels that results from the pumping action of the heart. Blood pressure is highest in the aorta and decreases as blood travels into smaller arteries, capillaries and then veins. Blood pressure exerts a force that causes a circumferential stretch of the vessel wall.
- Hyperlipidaemia
-
The state of blood carrying high levels of lipoproteins that contain cholesterol and triglycerides.
- Laplace's law
-
This law states that tension in the vessel wall equals the difference in pressure across the vessel times the radius of the vessel, divided by the thickness of the wall. Thus, higher blood pressure or vessels of larger radius require thicker walls to be mechanically stable.
- Nephron
-
The kidney consists of millions of these functional units. Each nephron begins with a glomerulus, in which blood is filtered through a specialized basement membrane. The resultant cell-free fluid enters a tube that is lined with epithelial cells that transport valuable components back into the blood, with the remainder excreted as urine.
- Foam cell
-
A macrophage in the artery wall that has become engorged with cholesterol esters and triglycerides.
- Apolipoprotein E
-
An important constituent of the high density lipoprotein, which carries 'good' cholesterol from the tissues to the liver.
- Vasorelaxation
-
The release of factors, such as nitric oxide, by the endothelium causes relaxation of the smooth muscle layer, leading to widening of the artery lumen.
Rights and permissions
About this article
Cite this article
Hahn, C., Schwartz, M. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol 10, 53–62 (2009). https://doi.org/10.1038/nrm2596
Issue Date:
DOI: https://doi.org/10.1038/nrm2596