Key Points
-
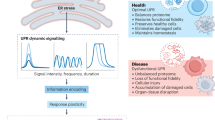
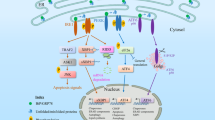
The endoplasmic reticulum (ER)-localized transmembrane proteins IRE1, PERK and ATF6 detect the load of unfolded protein in the lumen of the organelle, serving as stress receptors. They transduce the ER stress signal to the nucleus to regulate transcription and to the translational apparatus to regulate protein synthesis. Collectively, they signal the unfolded protein response (UPR).
-
Despite the unrelated mechanisms of downstream signalling, significant functional redundancy exists between the three known arms of the UPR. The transcriptional effects of the three arms also overlap significantly, which is achieved, in part, through mutual positive reinforcement.
-
The UPR reprogrammes transcription to enhance the capacity of the ER to cope with misfolded proteins, but also affects the function of the translocon and the repertoire of mRNA translated in the cell. Lipid synthesis is also integrated into the UPR to result in an expansion of the ER, which is especially prominent in professional secretory cells.
-
The UPR enhances the capacity of cells to degrade misfolded ER proteins by two parallel mechanisms: ER associated degradation, in which individual misfolded proteins are retro-translocated to the cytosol for proteasomal degradation, and autophagy — a less selective process in which segments of ER are engulfed by a limiting membrane and trafficked to the lysosome.
-
The three arms of the UPR have powerful pro-survival effects on ER-stressed cells. However, there are examples of failed homeostasis in which specific facets of UPR signalling contribute actively to the death of ER-stressed cells.
-
The pro-survival features of the UPR benefit cancer cells in the hypoxic cores of tumours, raising the possibility that inhibitors of IRE1, PERK or ATF6 might be useful as anti-cancer drugs.
Abstract
The endoplasmic reticulum (ER) responds to the accumulation of unfolded proteins in its lumen (ER stress) by activating intracellular signal transduction pathways — cumulatively called the unfolded protein response (UPR). Together, at least three mechanistically distinct arms of the UPR regulate the expression of numerous genes that function within the secretory pathway but also affect broad aspects of cell fate and the metabolism of proteins, amino acids and lipids. The arms of the UPR are integrated to provide a response that remodels the secretory apparatus and aligns cellular physiology to the demands imposed by ER stress.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kozutsumi, Y., Segal, M., Normington, K., Gething, M. J. & Sambrook, J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature 332, 462–464 (1988).
Schroder, M. & Kaufman, R. J. The mammalian unfolded protein response. Annu. Rev. Biochem. 74, 739–789 (2005).
Bernales, S., Papa, F. R. & Walter, P. Intracellular signaling by the unfolded protein response. Annu. Rev. Cell. Dev. Biol. 22, 487–508 (2006).
Cox, J. S., Shamu, C. E. & Walter, P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 73, 1197–1206 (1993).
Mori, K., Ma, W., Gething, M. J. & Sambrook, J. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell 74, 743–756 (1993). References 4 and 5 report the cloning of IRE1, the first component of the unfolded protein response to be identified.
Credle, J. J., Finer-Moore, J. S., Papa, F. R., Stroud, R. M. & Walter, P. On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proc. Natl Acad. Sci. USA 102, 18773–18784 (2005).
Zhou, J. et al. The crystal structure of human IRE1 luminal domain reveals a conserved dimerization interface required for activation of the unfolded protein response. Proc. Natl Acad. Sci. USA 103, 14343–14348 (2006). References 6 and 7 provide the first structural insight into the detection of the endoplasmic reticulum stress signal.
Shamu, C. E. & Walter, P. Oligomerization and phosphorylation of the Ire1p kinase during intracellular signaling from the endoplasmic reticulum to the nucleus. EMBO J. 15, 3028–3039 (1996).
Papa, F. R., Zhang, C., Shokat, K. & Walter, P. Bypassing a kinase activity with an ATP-competitive drug. Science 302, 1533–1537 (2003).
Cox, J. S. & Walter, P. A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell 87, 391–404 (1996).
Mori, K., Kawahara, T., Yoshida, H., Yanagi, H. & Yura, T. Signalling from endoplasmic reticulum to nucleus: transcription factor with a basic-leucine zipper motif is required for the unfolded protein-response pathway. Genes Cells 1, 803–817 (1996).
Yoshida, H., Matsui, T., Yamamoto, A., Okada, T. & Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107, 881–891 (2001).
Calfon, M. et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415, 92–96 (2002).
Sidrauski, C., Cox, J. S. & Walter, P. tRNA ligase is required for regulated mRNA splicing in the unfolded protein response. Cell 87, 405–413 (1996).
Stephens, S. B. et al. Stable ribosome binding to the endoplasmic reticulum enables compartment-specific regulation of mRNA translation. Mol. Biol. Cell 16, 5819–5831 (2005).
Ruegsegger, U., Leber, J. H. & Walter, P. Block of HAC1 mRNA translation by long-range base pairing is released by cytoplasmic splicing upon induction of the unfolded protein response. Cell 107, 103–114 (2001).
Yoshida, H., Oku, M., Suzuki, M. & Mori, K. pXBP1(U) encoded in XBP1 pre-mRNA negatively regulates unfolded protein response activator pXBP1(S) in mammalian ER stress response. J. Cell Biol. 172, 565–575 (2006).
Leber, J. H., Bernales, S. & Walter, P. IRE1-independent gain control of the unfolded protein response. PLoS Biol. 2, e235 (2004).
Niwa, M., Patil, C. K., DeRisi, J. & Walter, P. Genome-scale approaches for discovering novel nonconventional splicing substrates of the Ire1 nuclease. Genome Biol. 6, R3 (2005).
Nekrutenko, A. & He, J. Functionality of unspliced XBP1 is required to explain evolution of overlapping reading frames. Trends Genet. 22, 645–648 (2006).
Shen, X., Ellis, R. E., Sakaki, K. & Kaufman, R. J. Genetic interactions due to constitutive and inducible gene regulation mediated by the unfolded protein response in C. elegans. PLoS Genet. 1, e37 (2005).
Urano, F. et al. Coupling of stress in the endoplasmic reticulum to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287, 664–666 (2000).
Yoneda, T. et al. Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor-2- dependent mechanism in response to the ER stress. J. Biol. Chem. 276, 13935–13940 (2001).
Hetz, C. et al. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1α. Science 312, 572–576 (2006).
Nishitoh, H. et al. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 16, 1345–1355 (2002).
Hollien, J. & Weissman, J. S. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 313, 104–107 (2006).
Haze, K., Yoshida, H., Yanagi, H., Yura, T. & Mori, K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 10, 3787–3799 (1999). Reference 27 reports the identification of ATF6.
Ye, J. et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell 6, 1355–1364 (2000).
Shen, J., Chen, X., Hendershot, L. & Prywes, R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 and unmasking of Golgi localization signals. Dev. Cell 3, 99–111 (2002).
Zhang, K. et al. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell 124, 587–599 (2006).
Bertolotti, A., Zhang, Y., Hendershot, L., Harding, H. & Ron, D. Dynamic interaction of BiP and the ER stress transducers in the unfolded protein response. Nature Cell Biol. 2, 326–332 (2000).
Harding, H., Zhang, Y. & Ron, D. Translation and protein folding are coupled by an endoplasmic reticulum resident kinase. Nature 397, 271–274 (1999). Reference 32 reports the identification of PERK.
Harding, H. et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 11, 619–633 (2003).
Scheuner, D. et al. Translational control is required for the unfolded protein response and in-vivo glucose homeostasis. Mol. Cell 7, 1165–1176 (2001). Reference 34 reports the special role of eIF2a phosphorylation in preserving secretory cell viability and function.
Lu, P. D. et al. Cytoprotection by pre-emptive conditional phosphorylation of translation initiation factor 2. EMBO J. 23, 169–179 (2004).
Hinnebusch, A. G. & Natarajan, K. Gcn4p, a master regulator of gene expression, is controlled at multiple levels by diverse signals of starvation and stress. Eukaryot. Cell 1, 22–32 (2002).
Vattem, K. M. & Wek, R. C. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl Acad. Sci. USA 101, 11269–11274 (2004).
Lu, P. D., Harding, H. P. & Ron, D. Translation re-initiation at alternative open reading frames regulates gene expression in an integrated stress response. J. Cell Biol. 167, 27–33 (2004).
Jiang, H. Y. et al. Phosphorylation of the α subunit of eukaryotic initiation factor 2 is required for activation of NF-κB in response to diverse cellular stresses. Mol. Cell. Biol. 23, 5651–5663 (2003).
Deng, J. et al. Translational repression mediates activation of nuclear factor κB by phosphorylated translation initiation factor 2. Mol. Cell. Biol. 24, 10161–10168 (2004).
Jousse, C. et al. Inhibition of a constitutive translation initiation factor 2α phosphatase, CReP, promotes survival of stressed cells. J. Cell Biol. 163, 767–775 (2003).
Connor, J. H., Weiser, D. C., Li, S., Hallenbeck, J. M. & Shenolikar, S. Growth arrest and DNA damage-inducible protein GADD34 assembles a novel signaling complex containing protein phosphatase 1 and inhibitor 1. Mol. Cell. Biol. 21, 6841–6850 (2001).
Novoa, I., Zeng, H., Harding, H. & Ron, D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2α. J. Cell Biol. 153, 1011–1022 (2001).
Ma, Y. & Hendershot, L. M. Delineation of a negative feedback regulatory loop that controls protein translation during endoplasmic reticulum stress. J. Biol. Chem. 278, 34864–34873 (2003).
Brush, M. H., Weiser, D. C. & Shenolikar, S. Growth arrest and DNA damage-inducible protein GADD34 targets protein phosphatase 1α to the endoplasmic reticulum and promotes dephosphorylation of the α subunit of eukaryotic translation initiation factor 2. Mol. Cell. Biol. 23, 1292–1303 (2003).
Novoa, I. et al. Stress-induced gene expression requires programmed recovery from translational repression. EMBO J. 22, 1180–1187 (2003).
Harding, H. et al. Diabetes mellitus and exocrine pancreatic dysfunction in Perk−/− mice reveals a role for translational control in survival of secretory cells. Mol. Cell 7, 1153–1163 (2001).
Yoshida, H. et al. A time-dependent phase shift in the mammalian unfolded protein response. Dev. Cell 4, 265–271 (2003).
Patil, C. K., Li, H. & Walter, P. Gcn4p and novel upstream activating sequences regulate targets of the unfolded protein response. PLoS Biol. 2, e246 (2004).
Travers, K. J. et al. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 101, 249–258 (2000).
Murray, J. I. et al. Diverse and specific gene expression responses to stresses in cultured human cells. Mol. Biol. Cell 15, 2361–2374 (2004).
Harding, H. et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 6, 1099–1108 (2000). Reference 52 reports the role of eIF2a phosphorylation in regulating gene expression in the unfolded protein response.
Brodsky, J. L., Goeckeler, J. & Schekman, R. BiP and Sec63p are required for both co- and posttranslational protein translocation into the yeast endoplasmic reticulum. Proc. Natl Acad. Sci. USA 92, 9643–9646 (1995).
Kang, S.-W. et al. Substrate-specific translocational attenuation during ER stress defines a pre-emptive quality control pathway. Cell 127, 999–1013 (2006).
Cox, J. S., Chapman, R. E. & Walter, P. The unfolded protein response coordinates the production of endoplasmic reticulum protein and endoplasmic reticulum membrane. Mol. Biol. Cell 8, 1805–1814 (1997).
Reimold, A. M. et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature 412, 300–307 (2001).
Lee, A. H., Chu, G. C., Iwakoshi, N. N. & Glimcher, L. H. XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. EMBO J. 24, 4368–4380 (2005).
Shaffer, A. L. et al. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity 21, 81–93 (2004).
Sriburi, R., Jackowski, S., Mori, K. & Brewer, J. W. XBP1: a link between the unfolded protein response, lipid biosynthesis and biogenesis of the endoplasmic reticulum. J. Cell Biol. 167, 35–41 (2004). References 58 and 59 report the role of the unfolded protein response in the acquisition of a secretory cell phenotype.
Bernales, S., McDonald, K. L. & Walter, P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. 4, e423 (2006). Reference 60 reports the intersection of the unfolded protein response and autophagy.
van der Sanden, M. H., Houweling, M., van Golde, L. M. & Vaandrager, A. B. Inhibition of phosphatidylcholine synthesis induces expression of the endoplasmic reticulum stress and apoptosis-related protein CCAAT/enhancer-binding protein-homologous protein (CHOP/GADD153). Biochem. J. 369, 643–650 (2003).
Li, Y. et al. Enrichment of endoplasmic reticulum with cholesterol inhibits sarcoplasmic-endoplasmic reticulum calcium ATPase-2b activity in parallel with increased order of membrane lipids: implications for depletion of endoplasmic reticulum calcium stores and apoptosis in cholesterol-loaded macrophages. J. Biol. Chem. 279, 37030–37039 (2004).
Feng, B. et al. The endoplasmic reticulum as the site of cholesterol-induced cytotoxicity in macrophages. Nature Cell Biol. 5, 781–792 (2003).
Harding, H. P. et al. Bioactive small molecules reveal antagonism between the integrated stress response and sterol regulated gene expression. Cell Metab. 2, 361–371 (2005).
Zeng, L. et al. ATF6 modulates SREBP2-mediated lipogenesis. EMBO J. 23, 950–958 (2004).
Ozcan, U. et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 (2004).
Ozcan, U. et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313, 1137–1140 (2006).
Yorimitsu, T., Nair, U., Yang, Z. & Klionsky, D. J. Endoplasmic reticulum stress triggers autophagy. J. Biol. Chem. 281, 30299–30304 (2006).
Nakagawa, T. & Yuan, J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J. Cell Biol. 150, 887–894 (2000).
Wei, M. C. et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292, 727–730 (2001).
Nakagawa, T. et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature 403, 98–103 (2000).
Rutkowski, D. T. et al. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 4, e374 (2006).
Harding, H., Zhang, Y., Bertolotti, A., Zeng, H. & Ron, D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell 5, 897–904 (2000).
McCullough, K. D., Martindale, J. L., Klotz, L. O., Aw, T. Y. & Holbrook, N. J. GADD153 sensitizes cells to endoplasmic reticulum stress by down- regulating BCL2 and perturbing the cellular redox state. Mol. Cell. Biol. 21, 1249–1259 (2001).
Zinszner, H. et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 12, 982–995 (1998).
Oyadomari, S. et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J. Clin. Invest. 109, 525–532 (2002).
Marciniak, S. J. et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 18, 3066–3077 (2004).
Boyce, M. et al. A selective inhibitor of eIF2a dephosphorylation protects cells from ER stress. Science 307, 935–939 (2005).
Tan, S., Somia, N., Maher, P. & Schubert, D. Regulation of antioxidant metabolism by translation initiation factor 2α. J. Cell Biol. 152, 997–1006 (2001).
Silva, R. et al. CHOP/GADD153 is a mediator of apoptotic death in substantia nigra dopamine neurons in an in vivo neurotoxin model of parkinsonism. J. Neurochem. 95, 974–986 (2005).
Scheuner, D. et al. Double-stranded RNA-dependent protein kinase phosphorylation of the α-subunit of eukaryotic translation initiation factor 2 mediates apoptosis. J. Biol. Chem. 281, 21458–21468 (2006).
Fogli, A. et al. The effect of genotype on the natural history of eIF2B-related leukodystrophies. Neurology 62, 1509–1517 (2004).
Sitia, R. & Braakman, I. Quality control in the endoplasmic reticulum protein factory. Nature 426, 891–894 (2003).
Sekijima, Y. et al. The biological and chemical basis for tissue-selective amyloid disease. Cell 121, 73–85 (2005).
Okamura, K., Kimata, Y., Higashio, H., Tsuru, A. & Kohno, K. Dissociation of Kar2p/BiP from an ER sensory molecule, Ire1p, triggers the unfolded protein response in yeast. Biochem. Biophys. Res. Commun. 279, 445–450 (2000).
Kimata, Y., Oikawa, D., Shimizu, Y., Ishiwata-Kimata, Y. & Kohno, K. A role for BiP as an adjustor for the endoplasmic reticulum stress-sensing protein Ire1. J. Cell Biol. 167, 445–456 (2004).
Ma, Y. & Hendershot, L. M. The role of the unfolded protein response in tumour development: friend or foe? Nature Rev. Cancer 4, 966–977 (2004).
Queitsch, C., Sangster, T. A. & Lindquist, S. Hsp90 as a capacitor of phenotypic variation. Nature 417, 618–624 (2002).
Koumenis, C. et al. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2α. Mol. Cell. Biol. 22, 7405–7416 (2002).
Koritzinsky, M. et al. Gene expression during acute and prolonged hypoxia is regulated by distinct mechanisms of translational control. EMBO J. 25, 1114–1125 (2006).
Blais, J. D. et al. Activating transcription factor 4 is translationally regulated by hypoxic stress. Mol. Cell. Biol. 24, 7469–7482 (2004).
Bi, M. et al. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 24, 3470–3481 (2005).
Feldman, D. E., Chauhan, V. & Koong, A. C. The unfolded protein response: a novel component of the hypoxic stress response in tumors. Mol. Cancer Res. 3, 597–605 (2005).
Jamora, C., Dennert, G. & Lee, A. S. Inhibition of tumor progression by suppression of stress protein GRP78/BiP induction in fibrosarcoma B/C10ME. Proc. Natl Acad. Sci. USA 93, 7690–7694 (1996).
Blais, J. et al. PERK-dependent translational regulation promotes tumor cell adaptation and angiogenesis in response to hypoxic stress. Mol. Cell Biol. 26, 9517–9532 (2006).
Romero-Ramirez, L. et al. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res. 64, 5943–5947 (2004).
Sidrauski, C. & Walter, P. The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell 90, 1031–1039 (1997).
Marciniak, S. J., Garcia-Bonilla, L., Hu, J., Harding, H. P. & Ron, D. Activation-dependent substrate recruitment by the eukaryotic translation initiation factor 2 kinase PERK. J. Cell Biol. 172, 201–209 (2006).
Scheuner, D. et al. Control of mRNA translation preserves endoplasmic reticulum function in b cells and maintains glucose homeostasis. Nature Med. 11, 757–764 (2005).
Scorrano, L. et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science 300, 135–139 (2003).
Acknowledgements
We are indebted to our laboratory members and trainees who contributed ideas and experiments to many of the studies described here, and we apologize to those colleagues whose publications could not be cited owing to space limitations. Work in our laboratories is supported by the National Institutes of Health (D.R. and P.W.) and the Howard Hughes Medical Institute (P.W.).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- ER stress
-
The consequence of a mismatch between the load of unfolded and misfolded proteins in the endoplasmic reticulum and the capacity of the cellular machinery that copes with that load.
- Major histocompatibility complex
-
A plasma-membrane protein complex that binds and displays immunogenic peptides in a characteristic groove structure.
- Sterol response element binding protein
-
A protein from a family of ER-localized membrane-anchored transcription factors that are activated by sterol depletion to bind genes involved in sterol and fatty acid synthesis.
- Acute-phase responsive protein
-
A serum protein secreted by the liver and, to a lesser degree, by other cells in response to systemic inflammation.
- Uncharged transfer RNA
-
A transfer RNA that has not been amino-acylated by its (cognate) amino acid.
- Integrated stress response
-
The consequences of eIF2α phosphorylation on Ser51, which is effected by four different kinases that respond to diverse upstream stress signals, including ER stress.
- Amino-acid transporter
-
A plasma-membrane protein that transports amino acids into cells. Many are transcriptionally activated by ATF4 and, thus, are dependent on the activity of PERK.
- Translocon
-
A proteinaceous channel in the ER membrane, through which nascent proteins are translocated into the ER lumen.
- Chaperone reserve
-
The capacity of an organelle to tolerate a further load of unfolded proteins.
- Signal peptide
-
The portion of a secreted pro-protein that specifies translocation into the ER. It is usually cleaved off following translocation.
- Obesity
-
A metabolic state characterized by excessive accumulation of triglycerides in adipose tissue.
- ER-associated protein degradation
-
The process whereby misfolded ER proteins are delivered to the cytoplasm for proteasomal degradation.
- Autophagy
-
A collection of pathways by which sections of the cytoplasm, including the organelles suspended in it, are sequestered into membrane-bounded compartments that then fuse with lysosomes, where their content is degraded by acid hydrolases.
- Ischaemia
-
The metabolic consequences of inadequate blood supply. It is a common occurrence in large tumours and is associated with a poor response to treatment.
- Nude mouse
-
An immune-compromised mouse that tolerates tumour xenografts and is used to model tumour growth in a living animal.
Rights and permissions
About this article
Cite this article
Ron, D., Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8, 519–529 (2007). https://doi.org/10.1038/nrm2199
Issue Date:
DOI: https://doi.org/10.1038/nrm2199
This article is cited by
-
Integrated multi-omic analysis and experiment reveals the role of endoplasmic reticulum stress in lung adenocarcinoma
BMC Medical Genomics (2024)
-
Analysis of autophagy in DLBCL reveals subtype-specific differences and the preferential targeting of ULK1 inhibition in GCB-DLBCL provides a rationale as a new therapeutic approach
Leukemia (2024)
-
SPRTN is involved in hepatocellular carcinoma development through the ER stress response
Cancer Gene Therapy (2024)
-
Therapeutic potential of melatonin in targeting molecular pathways of organ fibrosis
Pharmacological Reports (2024)
-
Mzb1 Attenuates Atherosclerotic Plaque Vulnerability in ApoE-/- Mice by Alleviating Apoptosis and Modulating Mitochondrial Function
Journal of Cardiovascular Translational Research (2024)