Key Points
-
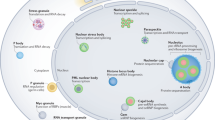
The primary function of the nucleolus is as the site of ribosome-subunit biogenesis in eukaryotic cells. The initial ribosomal RNA (rRNA) precursor is transcribed by RNA polymerase I and is subsequently processed and assembled with the many ribosomal proteins to form ribosome subunits, which are exported to the cytoplasm.
-
The nucleolus is a dynamic structure that disassembles when cells enter mitosis and reassembles following cell division. This involves a complex and highly regulated series of stepwise molecular assembly and disassembly pathways.
-
Nucleoli respond to changes in cellular growth rate and metabolic activity by altering rates of ribosome production, which indicates that they constantly receive and react to signalling events. Various proteins and activities have been shown to associate with the nucleolus specifically at different stages of the cell cycle, which suggests a role for nucleoli in regulating specific aspects of cell-cycle progression.
-
The nucleolus has been linked to several human diseases involving a range of different mechanisms. Multiple genetic disorders have been mapped to human genes that encode proteins that are known to associate with nucleoli, whereas many forms of cancer and viral infections affect nucleolar structure or the biogenesis of ribosomes.
-
As well as its role in coordinating the processing and maturation of rRNAs, several lines of evidence indicate that the nucleolus is also involved in the processing and/or maturation of additional classes of cellular ribonucleoproteins (RNPs), including the signal recognition particle and telomerase reverse transcriptase. This supports a role for the nucleolus as an important centre for RNP biogenesis.
Abstract
The nucleolus is a distinct subnuclear compartment that was first observed more than 200 years ago. Nucleoli assemble around the tandemly repeated ribosomal DNA gene clusters and 28S, 18S and 5.8S ribosomal RNAs (rRNAs) are transcribed as a single precursor, which is processed and assembled with the 5S rRNA into ribosome subunits. Although the nucleolus is primarily associated with ribosome biogenesis, several lines of evidence now show that it has additional functions. Some of these functions, such as regulation of mitosis, cell-cycle progression and proliferation, many forms of stress response and biogenesis of multiple ribonucleoprotein particles, will be discussed, as will the relation of the nucleolus to human diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Fatica, A. & Tollervey, D. Making ribosomes. Curr. Opin. Cell Biol. 14, 313–318 (2002).
Tschochner, H. & Hurt, E. Pre-ribosomes on the road from the nucleolus to the cytoplasm. Trends Cell Biol. 13, 255–263 (2003).
Andersen, J. S. et al. Directed proteomic analysis of the human nucleolus. Curr. Biol. 12, 1–11 (2002).
Pendle, A. F. et al. Proteomic analysis of the Arabidopsis nucleolus suggests novel nucleolar functions. Mol. Biol. Cell 16, 260–269 (2005).
Scherl, A. et al. Functional proteomic analysis of human nucleolus. Mol. Biol. Cell 13, 4100–4109 (2002).
Andersen, J. S. et al. Nucleolar proteome dynamics. Nature 433, 77–83 (2005). A quantitative proteomic approach for the temporal characterization of protein flux through the nucleolus in response to transcription and proteasome inhibitors.
Leung, A. K., Andersen, J. S., Mann, M. & Lamond, A. I. Bioinformatic analysis of the nucleolus. Biochem. J. 376, 553–569 (2003).
Coute, Y. et al. Deciphering the human nucleolar proteome. Mass Spectrom. Rev. 25, 215–234 (2006).
Hinsby, A. M. et al. A wiring of the human nucleolus. Mol. Cell 22, 285–295 (2006).
Heix, J. et al. Mitotic silencing of human rRNA synthesis: inactivation of the promoter selectivity factor SL1 by cdc2/cyclin B-mediated phosphorylation. EMBO J. 17, 7373–7381 (1998).
Leung, A. K. et al. Quantitative kinetic analysis of nucleolar breakdown and reassembly during mitosis in live human cells. J. Cell Biol. 166, 787–800 (2004). The authors characterize the reproducible and defined temporal order in which nucleolar components reassemble after mitosis.
Roussel, P., Andre, C., Comai, L. & Hernandez-Verdun, D. The rDNA transcription machinery is assembled during mitosis in active NORs and absent in inactive NORs. J. Cell Biol. 133, 235–246 (1996).
Dundr, M., Misteli, T. & Olson, M. O. The dynamics of postmitotic reassembly of the nucleolus. J. Cell Biol. 150, 433–446 (2000).
Gautier, T., Robert-Nicoud, M., Guilly, M. N. & Hernandez-Verdun, D. Relocation of nucleolar proteins around chromosomes at mitosis. A study by confocal laser scanning microscopy. J. Cell Sci. 102, 729–737 (1992).
Dimario, P. J. Cell and molecular biology of nucleolar assembly and disassembly. Int. Rev. Cytol. 239, 99–178 (2004).
Dundr, M. et al. Location of the HIV-1 Rev protein during mitosis: inactivation of the nuclear export signal alters the pathway for postmitotic reentry into nucleoli. J. Cell Sci. 109, 2239–2251 (1996).
Sirri, V., Roussel, P. & Hernandez-Verdun, D. In vivo release of mitotic silencing of ribosomal gene transcription does not give rise to precursor ribosomal RNA processing. J. Cell Biol. 148, 259–270 (2000).
Savino, T. M., Gebrane-Younes, J., De Mey, J., Sibarita, J. B. & Hernandez-Verdun, D. Nucleolar assembly of the rRNA processing machinery in living cells. J. Cell Biol. 153, 1097–1110 (2001). A directional and dynamic nuclear flow of proteins is described both between PNBs and between PNBs and nucleoli.
Hernandez-Verdun, D. Nucleolus: from structure to dynamics. Histochem. Cell Biol. 125, 127–137 (2006).
Angelier, N. et al. Tracking the interactions of rRNA processing proteins during nucleolar assembly in living cells. Mol. Biol. Cell 16, 2862–2871 (2005).
Sirri, V., Hernandez-Verdun, D. & Roussel, P. Cyclin-dependent kinases govern formation and maintenance of the nucleolus. J. Cell Biol. 156, 969–981 (2002).
Visintin, R. & Amon, A. The nucleolus: the magician's hat for cell cycle tricks. Curr. Opin. Cell Biol. 12, 752 (2000).
Kroetz, M. B. SUMO: a ubiquitin-like protein modifier. Yale J. Biol. Med. 78, 197–201 (2005).
Johnson, E. S. Protein modification by SUMO. Annu. Rev. Biochem. 73, 355–382 (2004).
Gong, L. & Yeh, E. T. Characterization of a family of nucleolar SUMO-specific proteases with preference for SUMO-2 or SUMO-3. J. Biol. Chem. 281, 15869–15877 (2006).
Di Bacco, A. et al. The SUMO-specific protease SENP5 is required for cell division. Mol. Cell. Biol. 26, 4489–4498 (2006).
Visintin, R. et al. The phosphatase Cdc14 triggers mitotic exit by reversal of Cdk-dependent phosphorylation. Mol. Cell 2, 709–718 (1998).
Shou, W. et al. Exit from mitosis is triggered by Tem1-dependent release of the protein phosphatase Cdc14 from nucleolar RENT complex. Cell 97, 233–244 (1999).
Azzam, R. et al. Phosphorylation by cyclin B–Cdk underlies release of mitotic exit activator Cdc14 from the nucleolus. Science 305, 516–519 (2004).
D'Amours, D., Stegmeier, F. & Amon, A. Cdc14 and condensin control the dissolution of cohesin-independent chromosome linkages at repeated DNA. Cell 117, 455–469 (2004).
Ceulemans, H. & Bollen, M. Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiol. Rev. 84, 1–39 (2004).
Andreassen, P. R., Lacroix, F. B., Villa-Moruzzi, E. & Margolis, R. L. Differential subcellular localization of protein phosphatase-1α, γ1, and δ isoforms during both interphase and mitosis in mammalian cells. J. Cell Biol. 141, 1207–1215 (1998).
Trinkle-Mulcahy, L., Sleeman, J. E. & Lamond, A. I. Dynamic targeting of protein phosphatase 1 within the nuclei of living mammalian cells. J. Cell Sci. 114, 4219–4228 (2001).
Trinkle-Mulcahy, L., Chusainow, J., Lam, Y. W., Swift, S. & Lamond, A. Visualization of intracellular pp1 targeting through transiently and stably expressed fluorescent protein fusions. Methods Mol. Biol. 365, 133–154 (2006).
Vagnarelli, P. et al. Condensin and Repo-Man–PP1 co-operate in the regulation of chromosome architecture during mitosis. Nature Cell Biol. 8, 1133–1142 (2006).
Trinkle-Mulcahy, L. & Lamond, A. I. Mitotic phosphatases: no longer silent partners. Curr. Opin. Cell Biol. 18, 623–631 (2006).
Wong, J. M., Kusdra, L. & Collins, K. Subnuclear shuttling of human telomerase induced by transformation and DNA damage. Nature Cell Biol. 4, 731–736 (2002).
Khurts, S. et al. Nucleolin interacts with telomerase. J. Biol. Chem. 279, 51508–51515 (2004).
Prives, C. Signaling to p53: breaking the MDM2–p53 circuit. Cell 95, 5–8 (1998).
Wsierska-Gadek, J. & Horky, M. How the nucleolar sequestration of p53 protein or its interplayers contributes to its (re)-activation. Ann. NY Acad. Sci. 1010, 266–272 (2003).
Bertwistle, D., Sugimoto, M. & Sherr, C. J. Physical and functional interactions of the Arf tumor suppressor protein with nucleophosmin/B23. Mol. Cell. Biol. 24, 985–996 (2004).
Olson, M. O. J. Sensing cellular stress: another new function for the nucleolus? Sci. STKE 224, pe 10 (2004).
Mayer, C., Bierhoff, H. & Grummt, I. The nucleolus as a stress sensor: JNK2 inactivates the transcription factor TIF-IA and down-regulates rRNA synthesis. Genes Dev. 19, 933–941 (2005).
Marciniak, R. A., Lombard, D. B., Johnson, F. B. & Guarente, L. Nucleolar localization of the Werner syndrome protein in human cells. Proc. Natl Acad. Sci. USA 95, 6887–6892 (1998).
Brosh, R. M. Jr et al. p53 Modulates the exonuclease activity of Werner syndrome protein. J. Biol. Chem. 276, 35093–35102 (2001).
Isaac, C. et al. Characterization of the nucleolar gene product, treacle, in Treacher Collins syndrome. Mol. Biol. Cell 11, 3061–3071 (2000).
Heiss, N. S. et al. Dyskerin localizes to the nucleolus and its mislocalization is unlikely to play a role in the pathogenesis of dyskeratosis congenita. Hum. Mol. Genet. 8, 2515–2524 (1999).
Woo, L. L., Futami, K., Shimamoto, A., Furuichi, Y. & Frank, K. M. The Rothmund–Thomson gene product RECQL4 localizes to the nucleolus in response to oxidative stress. Exp. Cell Res. 312, 3443–3457 (2006).
Bachrati, C. Z. & Hickson, I. D. RecQ helicases: suppressors of tumorigenesis and premature aging. Biochem. J. 374, 577–606 (2003).
Yankiwski, V., Marciniak, R. A., Guarente, L. & Neff, N. F. Nuclear structure in normal and Bloom syndrome cells. Proc. Natl Acad. Sci. USA 97, 5214–5219 (2000).
Werner, S. R., Prahalad, A. K., Yang, J. & Hock, J. M. RECQL4-deficient cells are hypersensitive to oxidative stress/damage: insights for osteosarcoma prevalence and heterogeneity in Rothmund–Thomson syndrome. Biochem. Biophys. Res. Commun. 345, 403–409 (2006).
Moseley, J. M. et al. Parathyroid hormone-related protein purified from a human lung cancer cell line. Proc. Natl Acad. Sci. USA 84, 5048–5052 (1987).
Lam, M. H., Hu, W., Xiao, C. Y., Gillespie, M. T. & Jans, D. A. Molecular dissection of the importin β1-recognized nuclear targeting signal of parathyroid hormone-related protein. Biochem. Biophys. Res. Commun. 282, 629–634 (2001).
Henderson, J. E. et al. Nucleolar localization of parathyroid hormone-related peptide enhances survival of chondrocytes under conditions that promote apoptotic cell death. Mol. Cell. Biol. 15, 4064–4075 (1995).
Dittmer, A. et al. Parathyroid hormone-related protein regulates tumor-relevant genes in breast cancer cells. J. Biol. Chem. 281, 14563–14572 (2006).
Choesmel, V. et al. Impaired ribosome biogenesis in Diamond–Blackfan anemia. Blood 109, 1275–1283 (2007). RPS19 is shown to have an essential role in biogenesis of the 40S small ribosome subunit in human cells.
Wang, C., Query, C. C. & Meier, U. T. Immunopurified small nucleolar ribonucleoprotein particles pseudouridylate rRNA independently of their association with phosphorylated Nopp140. Mol. Cell. Biol. 22, 8457–8466 (2002).
Heiss, N. S. et al. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nature Genet. 19, 32–38 (1998).
Mitchell, J. R., Wood, E. & Collins, K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature 402, 551–555 (1999).
Montanaro, L. et al. Dyskerin expression influences the level of ribosomal RNA pseudo-uridylation and telomerase RNA component in human breast cancer. J. Pathol. 210, 10–18 (2006).
Ruggero, D. et al. Dyskeratosis congenita and cancer in mice deficient in ribosomal RNA modification. Science 299, 259–262 (2003). Hypomorphic Dkc1 mutant ( Dkc1 m) mice recapitulate the clinical features of dyskeratosis congenita. Dkc1 m cells were impaired in rRNA pseudouridylation before the onset of disease.
Ruggero, D. & Pandolfi, P. P. Does the ribosome translate cancer? Nature Rev. Cancer 3, 179–192 (2003).
Grandori, C. et al. c-Myc binds to human ribosomal DNA and stimulates transcription of rRNA genes by RNA polymerase I. Nature Cell Biol. 7, 311–318 (2005).
Arabi, A. et al. c-Myc associates with ribosomal DNA and activates RNA polymerase I transcription. Nature Cell Biol. 7, 303–310 (2005).
Kondo, T. et al. Identification and characterization of nucleophosmin/B23/numatrin which binds the anti-oncogenic transcription factor IRF-1 and manifests oncogenic activity. Oncogene 15, 1275–1281 (1997).
Grisendi, S. et al. Role of nucleophosmin in embryonic development and tumorigenesis. Nature 437, 147–153 (2005).
Naoe, T., Suzuki, T., Kiyoi, H. & Urano, T. Nucleophosmin: a versatile molecule associated with hematological malignancies. Cancer Sci. 97, 963–969 (2006).
Ochs, R. L., Stein, T. W. Jr & Tan, E. M. Coiled bodies in the nucleolus of breast cancer cells. J. Cell Sci. 107, 385–399 (1994).
Aydin, H., Zhou, M., Herawi, M. & Epstein, J. I. Number and location of nucleoli and presence of apoptotic bodies in diagnostically challenging cases of prostate adenocarcinoma on needle biopsy. Hum. Pathol. 36, 1172–1177 (2005).
Adeyemi, B. F., Kolude, B. M., Akang, E. E. & Lawoyin, J. O. A study of the utility of silver nucleolar organizer regions in categorization and prognosis of salivary gland tumors. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 102, 513–520 (2006).
Dove, B. K. et al. Changes in nucleolar morphology and proteins during infection with the coronavirus infectious bronchitis virus. Cell Microbiol. 8, 1147–1157 (2006).
Hiscox, J. A. The nucleolus — a gateway to viral infection? Arch. Virol. 147, 1077–1089 (2002).
Ryabov, E. V., Kim, S. H. & Taliansky, M. Identification of a nuclear localization signal and nuclear export signal of the umbraviral long-distance RNA movement protein. J. Gen. Virol. 85, 1329–1333 (2004).
Kim, S. H., Ryabov, E. V., Brown, J. W. & Taliansky, M. Involvement of the nucleolus in plant virus systemic infection. Biochem. Soc. Trans. 32, 557–560 (2004).
Hatanaka, M. Discovery of the nucleolar targeting signal. Bioessays 12, 143–148 (1990).
Bevington, J. M. et al. Adeno-associated virus interactions with B23/nucleophosmin: identification of sub-nucleolar virion regions. Virology 357, 102–113 (2007).
Fankhauser, C., Izaurralde, E., Adachi, Y., Wingfield, P. & Laemmli, U. K. Specific complex of human immunodeficiency virus type 1 Rev and nucleolar B23 proteins: dissociation by the Rev response element. Mol. Cell. Biol. 11, 2567–2575 (1991).
Michienzi, A., De Angelis, F. G., Bozzoni, I. & Rossi, J. J. A nucleolar localizing Rev binding element inhibits HIV replication. AIDS Res. Ther. 3, 13 (2006).
Donmez-Altuntas, H. et al. Evaluation of the nucleolar organizer regions in Alzheimer's disease. Gerontology 51, 297–301 (2005).
Wills, N. M. & Atkins, J. F. The potential role of ribosomal frameshifting in generating aberrant proteins implicated in neurodegenerative diseases. RNA 12, 1149–1153 (2006).
De Rooij, K. E., Dorsman, J. C., Smoor, M. A., Den Dunnen, J. T. & Van Ommen, G. J. Subcellular localization of the Huntington's disease gene product in cell lines by immunofluorescence and biochemical subcellular fractionation. Hum. Mol. Genet. 5, 1093–1099 (1996).
Gerbi, S. A., Borovjagin, A. V. & Lange, T. S. The nucleolus: a site of ribonucleoprotein maturation. Curr. Opin. Cell Biol. 15, 318–325 (2003).
Kiss, T. Small nucleolar RNAs: an abundant group of noncoding RNAs with diverse cellular functions. Cell 109, 145–148 (2002).
Wang, H., Boisvert, D., Kim, K. K., Kim, R. & Kim, S. H. Crystal structure of a fibrillarin homologue from Methanococcus jannaschii, a hyperthermophile, at 1.6 Å resolution. EMBO J. 19, 317–323 (2000).
Henras, A. K., Capeyrou, R., Henry, Y. & Caizergues-Ferrer, M. Cbf5p, the putative pseudouridine synthase of H/ACA-type snoRNPs, can form a complex with Gar1p and Nop10p in absence of Nhp2p and box H/ACA snoRNAs. RNA 10, 1704–1712 (2004).
Li, L. & Ye, K. Crystal structure of an H/ACA box ribonucleoprotein particle. Nature 443, 302–307 (2006).
Walter, P. & Johnson, A. E. Signal sequence recognition and protein targeting to the endoplasmic reticulum membrane. Annu. Rev. Cell Biol. 10, 87–119 (1994).
Jacobson, M. R. & Pederson, T. Localization of signal recognition particle RNA in the nucleolus of mammalian cells. Proc. Natl Acad. Sci. USA 95, 7981–7986 (1998).
Jacobson, M. R. et al. Nuclear domains of the RNA subunit of RNase P. J. Cell Sci. 110, 829–837 (1997).
Ganot, P., Jady, B. E., Bortolin, M. L., Darzacq, X. & Kiss, T. Nucleolar factors direct the 2′-O-ribose methylation and pseudouridylation of U6 spliceosomal RNA. Mol. Cell. Biol. 19, 6906–6917 (1999).
Desterro, J. M. et al. Dynamic association of RNA-editing enzymes with the nucleolus. J. Cell Sci. 116, 1805–1818 (2003).
Vitali, P. et al. ADAR2-mediated editing of RNA substrates in the nucleolus is inhibited by C/D small nucleolar RNAs. J. Cell Biol. 169, 745–753 (2005).
Li, C. F. et al. An ARGONAUTE4-containing nuclear processing center colocalized with Cajal bodies in Arabidopsis thaliana. Cell 126, 93–106 (2006).
Pontes, O. et al. The Arabidopsis chromatin-modifying nuclear siRNA pathway involves a nucleolar RNA processing center. Cell 126, 79–92 (2006).
Politz, J. C., Zhang, F. & Pederson, T. MicroRNA-206 colocalizes with ribosome-rich regions in both the nucleolus and cytoplasm of rat myogenic cells. Proc. Natl Acad. Sci. USA 103, 18957–18962 (2006). miR-206 was found to be localized in the cytoplasm and the nucleolus, which suggests that miR-206 can associate both with nascent ribosomes in the nucleolus and with exported, functional ribosomes in the cytoplasm.
Mais, C., Wright, J. E., Prieto, J. L., Raggett, S. L. & McStay, B. UBF-binding site arrays form pseudo-NORs and sequester the RNA polymerase I transcription machinery. Genes Dev. 19, 50–64 (2005).
McStay, B. Nucleolar dominance: a model for rRNA gene silencing. Genes Dev. 20, 1207–1214 (2006).
Acknowledgements
We are grateful to B. McStay for providing FISH images and D.P. Bazett-Jones for EF-TEM images. We thank Y. Wah Lam and other members of the Lamond group for advice and for providing images. A.I.L. is a Wellcome Trust Principal Research Fellow. F.-M.B. is supported by a fellowship from the Caledonian Research Foundation and S.V.K. by a fellowship from the Netherlands Organization for Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Acrocentric chromosome
-
A chromosome with a centromere that is located near one end of the chromosome. Humans have five pairs of acrocentric chromosomes.
- Small nucleolar ribonucleoproteins
-
Nucleolar RNA–protein complexes that function in pre-ribosomal RNA processing.
- CENP proteins
-
Proteins that associate with the centromere, the region of a chromosome that is attached to the spindle during nuclear division.
- Chromosomal passenger protein
-
A protein that shares a characteristic pattern of association with chromatin in prophase, centromeres in metaphase and early anaphase, and the midzone and midbody in late anaphase and telophase, respectively.
- Cdc14 early anaphase release (FEAR) network
-
A signalling network in which the role for the protein phosphatase Cdc14 is key in the coordination of the multiple events that occur during anaphase, such as partitioning of the DNA, regulation of spindle stability, activation of microtubule forces and initiation of mitotic exit.
- Werner syndrome
-
A rare autosomal recessive disorder, characterized by the early development of various age-related diseases. The gene that is responsible for Werner syndrome (WRN) encodes a DNA helicase that is homologous to Escherichia coli RecQ.
- Bloom syndrome
-
An autosomal recessive disorder that is characterized by growth deficiency, unusual facial features, sun sensitivity, telangiectatic erythema, immunodeficiency and a predisposition to cancer. BLM, the gene that is mutated in Bloom syndrome, encodes a DNA helicase of the RECQ family.
- Rothmund–Thomson syndrome
-
(RTS). Patients exhibit chromosome fragility, skin and skeletal defects, cataracts and an increased predisposition to osteosarcoma. Some cases of RTS are caused by mutations in the DNA helicase gene RECQL4.
- Promyelocytic leukaemia nuclear body
-
A round nuclear structure that contains several proteins, including the promyelocytic leukaemia protein (PML). It is thought to be the site of recruitment of various proteins and might also have a role in gene transcription.
- Cajal body
-
A round nuclear structure that contains several proteins, including coilin and survival of motor neuron (SMN1). It is thought to be the site of small nuclear ribonucleoprotein assembly and small nuclear RNA maturation.
- Small nuclear RNPs
-
Nuclear RNA–protein complexes that combine with pre-mRNA and various proteins to form the spliceosomes.
- Signal recognition particle
-
A ribonucleoprotein complex that is responsible for the recognition of the N-terminal signal-peptide sequence on nascent proteins and for the proper targeting of proteins onto a receptor on the cytoplasmic face of the endoplasmic reticulum.
Rights and permissions
About this article
Cite this article
Boisvert, FM., van Koningsbruggen, S., Navascués, J. et al. The multifunctional nucleolus. Nat Rev Mol Cell Biol 8, 574–585 (2007). https://doi.org/10.1038/nrm2184
Issue Date:
DOI: https://doi.org/10.1038/nrm2184
This article is cited by
-
Coronin 2B deficiency induces nucleolar stress and neuronal apoptosis
Cell Death & Disease (2024)
-
Micropolarity governs the structural organization of biomolecular condensates
Nature Chemical Biology (2024)
-
Genetic epidemiology of Woodhouse-Sakati Syndrome in the Greater Middle East region and beyond: a systematic review
Orphanet Journal of Rare Diseases (2023)
-
Quantitative real-time in-cell imaging reveals heterogeneous clusters of proteins prior to condensation
Nature Communications (2023)
-
Mapping nucleolus-associated chromatin interactions using nucleolus Hi-C reveals pattern of heterochromatin interactions
Nature Communications (2023)