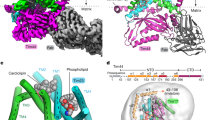
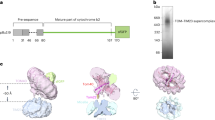
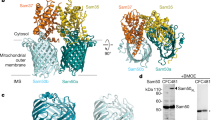
Key Points
-
The membrane-embedded multiprotein complexes of mitochondria mediate the transport of nuclear-encoded proteins across and into the outer or inner mitochondrial membranes.
-
The TOM (translocase of the outer mitochondrial membrane) complex consists of cytosol-exposed receptors and a pore-forming core, and it mediates the transport of proteins from the cytosol across and into the outer mitochondrial membrane. A novel protein complex in the outer membrane of mitochondria, called the SAM complex (sorting and assembly machinery), is involved in the biogenesis of β-barrel proteins of the outer membrane.
-
Two translocases of the inner mitochondrial membrane (TIM complexes) mediate protein transport at the inner membrane. The TIM23 complex (a presequence translocase) mediates the transport of presequence-containing proteins across and into the inner membrane. The TIM22 complex (a twin-pore carrier translocase) catalyses the insertion of multispanning proteins that have internal targeting signals into the inner membrane.
-
The TIM23 complex requires the PAM complex (presequence-translocase-associated motor complex) and a membrane potential
(Δψ) for the complete transport of proteins into the mitochondrial matrix. The insertion of presequence-containing inner-membrane proteins can be mediated by the TIM23 complex alone or also requires PAM in cases in which the protein enters the matrix space first and is exported into the inner membrane.
-
The TIM22 complex mediates the membrane insertion of multispanning inner-membrane proteins that have internal targeting signals, and it uses a Δψ as an external driving force. Membrane insertion by the TIM22 complex is a multistep process, in which the preprotein initially tethers to the translocase in a step that is independent of Δψ. Subsequently, in steps that require a Δψ, positive charges in the matrix-exposed loops of the precursor protein allow docking in the twin-pore translocase and, eventually, the precursor inserts into the inner membrane.
Abstract
The mitochondrial inner membrane is rich in multispanning integral membrane proteins, most of which mediate the vital transport of molecules between the matrix and the intermembrane space. The correct transport and membrane insertion of such proteins is essential for maintaining the correct exchange of molecules between mitochondria and the rest of the cell. Mitochondria contain several specific complexes — known as translocases — that translocate precursor proteins. Recent analysis of the inner-membrane, twin-pore protein translocase (TIM22 complex) allows a glimpse of the molecular mechanisms by which this machinery triggers protein insertion using the membrane potential as an external driving force.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sickmann, A. et al. The proteome of Saccharomyces cerevisiae mitochondria. Proc. Natl Acad. Sci. USA 100, 13207–13212 (2003).
Taylor, S. W. et al. Characterization of the human heart mitochondrial proteome. Nature Biotechnol. 21, 281–286 (2003). References 1 and 2 report the most comprehensive analyses so far of the mitochondrial proteome. These studies covered about 90% and 45% of the predicted mitochondrial proteomes of budding yeast and human heart, respectively.
Westermann, B. & Neupert, W. 'Omics' of the mitochondrion. Nature Biotechnol. 21, 239–240 (2003).
Truscott, K. N., Brandner, K. & Pfanner, N. Mechanisms of protein import into mitochondria. Curr. Biol. 13, R326–R337 (2003).
Schnell, D. J. & Hebert, D. N. Protein translocons. Multifunctional mediators of protein translocation across membranes. Cell 112, 491–505 (2003).
Holroyd, C. & Erdmann, R. Protein translocation machineries of peroxisomes. FEBS Lett. 501, 6–10 (2001).
Robinson, C., Thompson, S. J. & Woolhead, C. Multiple pathways used for the targeting of thylakoid proteins in chloroplasts. Traffic 2, 245–251 (2001).
Soll, J. & Schleiff, E. Protein import into chloroplasts. Nature Rev. Mol. Cell Biol. 5, 198–208 (2004).
Pfanner, N. & Geissler, A. Versatility of the mitochondrial protein import machinery. Nature Rev. Mol. Cell Biol. 2, 339–349 (2001).
Neupert, W. Protein import into mitochondria. Annu. Rev. Biochem. 66, 863–917 (1997).
Jensen, R. E. & Dunn, C. D. Protein import into and across the mitochondrial inner membrane: role of the TIM23 and TIM22 translocons. Biochim. Biophys. Acta 1592, 25–34 (2002).
Koehler, C. M., Merchant, S. & Schatz, G. How membrane proteins travel across the mitochondrial intermembrane space. Trends Biochem. Sci. 24, 428–432 (1999).
Endo, T. & Kohda, D. Functions of outer membrane receptors in mitochondrial protein import. Biochim. Biophys. Acta 1592, 3–14 (2002).
Abe, Y. et al. Structural basis of presequence recognition by the mitochondrial protein import receptor Tom20. Cell 100, 551–560 (2000).
Künkele, K. P. et al. The preprotein translocation channel of the outer membrane of mitochondria. Cell 93, 1009–1019 (1998).
Athing, U. et al. The TOM core complex: the general protein import pore of the outer membrane of mitochondria. J. Cell. Biol. 147, 959–968 (1999).
Model, K. et al. Protein translocase of the outer mitochondrial membrane: role of import receptors in the structural organization of the TOM complex. J. Mol. Biol. 316, 657–666 (2002).
Hill, K. et al. Tom40 forms the hydrophilic channel of the mitochondrial import pore for preproteins. Nature 395, 516–521 (1998).
Esaki, M. et al. Tom40 protein import channel binds to non-native proteins and prevents their aggregation. Nature Struct. Biol. 10, 988–994 (2003).
Horwich, A., Kalousek, F., Mellman, I. & Rosenberg, L. E. A leader peptide is sufficent to direct import of a chimeric protein. EMBO J. 4, 1129–1135 (1985).
Hurt, E. C., Pesold-Hurt, B. & Schatz, G. The cleavable prepiece of an imported mitochondrial protein is sufficient to direct cytosolic dihydrofolate reductase into the mitochondrial matrix. FEBS Lett. 178, 306–310 (1984).
Rehling, P., Pfanner, N. & Meisinger, C. Insertion of hydrophobic membrane proteins into the inner mitochondrial membrane — a guided tour. J. Mol. Biol. 326, 639–657 (2003).
Brix, J., Rüdiger, S., Bukau, B., Schneider-Mergener, J. & Pfanner, N. Distribution of binding sequences for the mitochondrial import receptors Tom20, Tom22, Tom70 in a presequence-carrying and a non-cleavable protein. J. Biol. Chem. 274, 16522–16530 (1999).
Wiedemann, N., Pfanner, N. & Ryan, M. T. The three modules of ADP/ATP carrier cooperate in receptor recruitment and translocation into mitochondria. EMBO J. 20, 951–960 (2001).
Brix, J. et al. The mitochondrial import receptor Tom 70: identification of a 25 kDa core domain with a specific binding site for preproteins. J. Mol. Biol. 303, 479–488 (2000).
Bolliger, L., Junne, T., Schatz, G. & Lithgow, T. Acidic receptor domains on both sides of the outer membrane mediate translocation of precursor proteins into yeast mitochondria. EMBO J. 14, 6318–6326 (1995).
Moczko, M. et al. The intermembrane space domain of mitochondrial Tom22 functions as a trans binding site for preproteins with N-terminal targeting sequences. Mol. Cell. Biol. 17, 6574–6584 (1997).
Emtage, J. L. T. & Jensen, R. E. MAS6 encodes an essential inner membrane component of the yeast mitochondrial protein import pathway. J. Biol. Chem. 122, 1003–1012 (1993).
Kübrich, M. et al. The polytopic mitochondrial inner membrane proteins MIM17 and MIM23 operate at the same preprotein import site. FEBS Lett. 349, 222–228 (1994).
Geissler, A. et al. The mitochondrial presequence translocase: an essential role of Tim50 in directing preproteins to the import channel. Cell 111, 507–518 (2002).
Yamamoto, H. et al. Tim50 is a subunit of the TIM23 complex that links protein translocation across the outer and inner mitochondrial membranes. Cell 111, 519–528 (2002).
Mokranjac, D. et al. Tim50, a novel component of the TIM23 preprotein translocase of mitochondria. EMBO J. 22, 816–825 (2003). References 30–32 show that Tim50 is a new component of the presequence translocase and that it directs proteins from the outer membrane to the Tim23 pore in the inner membrane.
Bauer, M. F., Sirrenberg, C., Neupert, W. & Brunner, M. Role of Tim23 as voltage sensor and presequence receptor in protein import into mitochondria. Cell 87, 33–41 (1996).
Komiya, T. et al. Interaction of mitochondrial targeting signals with acidic receptor domains along the protein import pathway: evidence for the 'acid chain' hypothesis. EMBO J. 17, 3886–3898 (1998).
Truscott, K. N. et al. A presequence- and voltage-sensitive channel of the mitochondrial preprotein translocase formed by Tim23. Nature Struct. Biol. 8, 1074–1082 (2001).
Donzeau, M. et al. Tim23 links the inner and outer mitochondrial membranes. Cell 101, 401–412 (2000).
Chacinska, A. et al. Mitochondrial translocation contact sites: separation of dynamic and stabilizing elements in formation of a TOM–TIM–preprotein supercomplex. EMBO J. 22, 5370–5381 (2003).
Martin, J., Mahlke, K. & Pfanner, N. Role of an energized inner membrane in mitochondrial protein import: ΔΨ drives the movement of presequences. J. Biol. Chem. 266, 18051–18057 (1991).
Huang, S., Ratliff, K. S. & Matouschek, A. Protein unfolding by the mitochondrial membrane potential. Nature Struct. Biol. 9, 301–307 (2002).
Gakh, O., Cavadini, P. & Isaya, G. Mitochondrial processing peptidases. Biochim. Biophys. Acta 1592, 63–77 (2002).
Taylor, A. B. et al. Crystal structures of mitochondrial processing peptidase reveal the mode for specific cleavage of import signal sequences. Structure 9, 615–625 (2001).
Pfanner, N. & Neupert, W. Distinct steps in the import of ADP/ATP carrier into mitochondria. J. Biol. Chem. 262, 7528–7536 (1987).
Pfanner, N., Tropschug, M. & Neupert, W. Mitochondrial protein import: nucleoside triphosphates are involved in conferring import-competence to precursors. Cell 49, 815–823 (1987). References 42 and 43 show that the import of hydrophobic carrier proteins into mitochondria follows a different mechanism than the import of presequence-containing proteins. Five consecutive carrier transport stages from the cytosol to the inner mitochondrial membrane and their energetic requirements are defined.
Young, J. C., Hoogenraad, N. J. & Hartl, F. U. Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell 112, 41–50 (2003). Shows that Hsp70 and Hsp90 are involved in the transport of carrier proteins from the cytosol to the mitochondrial surface. The outer-membrane receptor Tom70 has a specific binding site for these chaperones.
Söllner, T., Pfaller, R., Griffiths, G., Pfanner, N. & Neupert, W. A mitochondrial import receptor for the ADP/ATP carrier. Cell 62, 107–115 (1990).
Ryan, M. T., Müller, H. & Pfanner, N. Functional staging of ADP/ATP carrier translocation across the outer mitochondrial membrane. J. Biol. Chem. 274, 20619–20627 (1999).
Curran, S. P., Leuenberger, D., Schmidt, E. & Koehler, C. M. The role of the Tim8p–Tim13p complex in a conserved import pathway for mitochondrial polytopic inner membrane proteins. J. Cell Biol. 158, 1017–1027 (2002).
Sirrenberg, C. et al. Carrier protein import into mitochondria mediated by the intermembrane proteins Tim10/Mars11 and Tim 12/Mrs5. Nature 391, 912–915 (1998).
Koehler, C. M. et al. Import of mitochondrial carriers mediated by essential proteins of the intermembrane space. Science 279, 369–373 (1998). References 48 and 49 show that the essential mitochondrial proteins Tim10 and Tim12 mediate the transport of carrier proteins across the intermembrane space.
Adam, A. et al. Tim9, a new component of the TIM22.54 translocase. EMBO J. 18, 313–319 (1999).
Koehler, C. M. et al. Tim9p, an essential partner subunit of Tim10p for the import of mitochondrial carrier proteins. EMBO J. 17, 6477–6486 (1998).
Vasiljev, A. et al. Reconstituted TOM core complex and Tim9/Tim10 complex of mitochondria are sufficient for translocation of the ADP/ATP carrier across membranes. Mol. Biol. Cell 15, 1445–1458 (2004).
Curran, S. P., Leuenberger, D., Oppliger, W. & Koehler, C. M. The Tim9p–Tim10p complex binds to the transmembrane domains of the ADP/ATP carrier. EMBO J. 21, 942–953 (2002).
Koehler, C. M., Leuenberger, D., Merchant, S., Renold, A. & Junne, T. Human deafness dystonia syndrome is a mitochondrial disease. Proc. Natl Acad. Sci. USA 96, 2141–2146 (1999).
Paschen, S. A. et al. The role of the TIM8–13 complex in the import of Tim23 into mitochondria. EMBO J. 19, 6392–6400 (2000).
Davis, A. J., Sepuri, N. B., Holder, J., Johnson, A. E. & Jensen, R. E. Two intermembrane space TIM complexes interact with different domains of Tim23p during its import into mitochondria. J. Cell Biol. 150, 1271–1282 (2000).
Wiedemann, N. et al. Biogenesis of the protein import channel Tom40 of the mitochondrial outer membrane: intermembrane space components are involved in an early stage of the assembly pathway. J. Biol. Chem. 279, 18188–18194 (2004).
Hoppins, S. C. & Nargang, F. E. The Tim8–Tim13 complex in Neurospora crassa functions in the assembly of proteins into both mitochondrial membranes. J. Biol. Chem. 279, 12396–12405 (2004).
Lutz, T., Neupert, W. & Herrmann, J. M. Import of small Tim proteins into the mitochondrial intermembrane space. EMBO J. 22, 4400–4408 (2003).
Allen, S., Lu, H., Thornton, D. & Tokatlidis, K. Juxtaposition of the two distal CX3C motifs via intrachain disulfide bonding is essential for the folding of Tim10. J. Biol. Chem. 278, 38505–38513 (2003).
Koehler, C. M. The small Tim proteins and the twin Cx(3)C motif. Trends Biochem. Sci. 29, 1–4 (2004).
Pfanner, N. & Neupert, W. Transport of proteins into mitochondria: a potassium diffusion potential is able to drive the import of ADP/ATP carrier. EMBO J. 4, 2819–2825 (1985).
Koehler, C. M. et al. Tim18p, a new subunit of the Tim22 complex that mediates insertion of imported proteins into the yeast mitochondrial inner membrane. Mol. Cell. Biol. 20, 1187–1193 (2000).
Kerscher, O., Sepuri, N. B. & Jensen, R. E. Tim18p is a new component of the Tim54p–Tim22p translocon in the mitochondrial inner membrane. Mol. Biol. Cell. 11, 103–116 (2000).
Kerscher, O., Holder, J., Srinivasan, M., Leung, R. S. & Jensen, R. The Tim54p–Tim22p complex mediates insertion of proteins into the mitochondrial inner membrane. J. Cell Biol. 139, 1663–1675 (1997).
Sirrenberg, C., Bauer, M. F., Guiard, B., Neupert, W. & Brunner, M. Import of carrier proteins into the mitochondrial inner membrane mediated by Tim22. Nature 384, 582–585 (1996). References 65 and 66 identify Tim22 and Tim54 as two inner mitochondrial membrane proteins that are involved in carrier-protein insertion into the inner membrane.
Kovermann, P. et al. Tim22, the essential core of the mitochondrial protein insertion complex, forms a voltage-activated and signal-gated channel. Mol. Cell 9, 363–373 (2002). This work shows that the Tim22 subunit of the carrier translocase (TIM22 complex) forms a voltage-activated pore in the inner mitochondrial membrane.
Rehling, P. et al. Protein insertion into the mitochondria inner membrane by a twin-pore translocase. Science 299, 1747–1751 (2003). This study shows that the carrier translocase (TIM22 complex) forms a twin-pore translocase that uses the membrane potential and three consecutive steps to insert multispanning proteins into the inner membrane.
Endres, M., Neupert, W. & Brunner, B. Transport of the ADP/ATP carrier of mitochondria from the TOM complex to the TIM22.54 complex. EMBO J. 18, 3214–3221 (1999).
Truscott, K. N. et al. Mitochondrial import of the ADP/ATP carrier: the essential TIM complex of the intermembrane space is required for precursor release from the TOM complex. Mol. Cell. Biol. 22, 7780–7789 (2002).
Káldi, K., Bauer, M. F., Sirrenberg, C., Neupert, W. & Brunner, M. Biogenesis of Tim23 and Tim17, integral components of the TIM machinery for matrix-targeted preproteins. EMBO J. 17, 1569–1576 (1998).
Davis, A. J., Ryan, K. R. & Jensen, R. E. Tim23p contains separate and distinct signals for targeting to mitochondria and insertion into the inner membrane. Mol. Biol. Cell. 9, 2577–2593 (1998).
Nelson, D. R., Felix, C. M. & Swanson, J. M. Highly conserved charge-pair networks in the mitochondrial carrier family. J. Mol. Biol. 277, 285–308 (1998).
Palmieri, L. et al. Identification and functions of new transporters in yeast mitochondria. Biochim. Biophys. Acta 1459, 363–369 (2000).
Pebay-Peyroula, E. et al. Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature 426, 39–44 (2003).
Pfanner, N., Hoeben, P., Tropschug, M. & Neupert, W. The carboxyl-terminal two-thirds of the ADP/ATP carrier polypeptide contains sufficient information to direct translocation into mitochondria. J. Biol. Chem. 262, 14851–14854 (1987).
Smagula, C. & Douglas, M. G. Mitochondrial import of the ADP/ATP carrier protein in Saccharomyces cerevisiae. Sequences required for receptor binding and membrane translocation. J. Biol. Chem. 263, 6783–6790 (1988).
Frazier, A. E. et al. Mitochondria use different mechanisms for transport of multispanning membrane proteins through the intermembrane space. Mol. Cell. Biol. 23, 7818–7828 (2003).
Mootha, V. K. et al. Integrated analysis of protein composition, tissue diversity, and gene regulation in mouse mitochondria. Cell 115, 629–640 (2003).
Wiedemann, N. et al. Machinery for protein sorting and assembly in the mitochondrial outer membrane. Nature 424, 565–571 (2003). Reports the identification of a new complex in the mitochondrial outer membrane that is required for the sorting and assembly of β-barrel proteins. Mas37 is shown to be a subunit of this SAM complex.
Kozjak, V. et al. An essential role of Sam50 in the protein sorting and assembly machinery of the mitochondrial outer membrane. J. Biol. Chem. 278, 48520–48523 (2003).
Paschen, S. A. et al. Evolutionary conservation of biogenesis of β-barrel membrane proteins. Nature 426, 862–866 (2003).
Gentle, I., Gabriel, K., Beech, P., Waller, R. & Lithgow, T. The Omp85 family of proteins is essential for outer membrane biogenesis in mitochondria and bacteria. J. Cell Biol. 164, 19–24 (2004). References 81–83 identify the second subunit of the SAM complex, Sam50 (Tob55/Omp85). The conservation of this subunit indicates that the insertion of β-barrel proteins into the outer membrane of bacteria and mitochondria occurs by a similar mechanism.
Milenkovic, D. et al. Sam35 of the mitochondrial protein sorting and assembly machinery is a peripheral outer membrane protein essential for cell viability. J. Biol. Chem. 279, 22781–22785 (2004).
Voulhoux, R., Bos, M. P., Geurtsen, J., Mols, M. & Tommassen, J. Role of a highly conserved bacterial protein in outer membrane protein assembly. Science 299, 262–265 (2003).
Model, K. et al. Multistep assembly of the protein import channel of the mitochondrial outer membrane. Nature Struct. Biol. 8, 361–370 (2001).
Kang, P. et al. Requirement for Hsp70 in the mitochondrial matrix for translocation and folding of precursor proteins. Nature 348, 137–143 (1990).
Scherer, P. E., Krieg, U. C., Hwang, S. T., Vestweber, D. & Schatz, G. A precursor protein partly translocated into yeast mitochondria is bound to a 70 kd mitochondrial stress protein. EMBO J. 9, 4315–4322 (1990).
Schneider, H. C. et al. Mitochondrial Hsp70/MIM44 complex facilitates protein import. Nature 371, 768–774 (1994).
Rassow, J. et al. Mitochondrial protein import: biochemical and genetic evidence for interaction of matrix Hsp70 and the inner membrane protein MIM44. J. Cell Biol. 127, 1547–1556 (1994).
Kronidou, N. G. et al. Dynamic interaction between Isp45 and mitochondrial Hsp70 in the protein import system of the yeast mitochondrial inner membrane. Proc. Natl Acad. Sci. USA 91, 12818–12822 (1994).
Voisine, C. et al. The protein import motor of mitochondria: unfolding and trapping of preproteins are distinct and separable functions of matrix Hsp70. Cell 97, 565–574 (1999).
Liu, Q., D'Silva, P., Walter, W., Marszalek, J. & Craig, E. A. Regulated cycling of mitochondrial Hsp70 at the protein import channel. Science 300, 139–141 (2003).
Truscott, K. N. et al. A J-protein is an essential subunit of the presequence translocase-associated protein import motor of mitochondria. J. Cell. Biol. 163, 707–713 (2003).
D'Silva, P. D., Schilke, B., Walter, W., Andrew, A. & Craig, E. A. J protein cochaperone of the mitochondrial inner membrane required for protein import into the mitochondrial matrix. Proc. Natl Acad. Sci. USA 100, 13839–13844 (2003).
Mokranjac, D., Sichting, M., Neupert, W. & Hell, K. Tim14, a novel key component of the import motor of the TIM23 protein translocase of mitochondria. EMBO J. 22, 4945–4956 (2003). References 94–96 identify Pam18 (Tim14), a DnaJ-like protein, as a novel constituent of the PAM complex. Pam18 cooperates with mtHsp70 in matrix-protein import and is required for the reaction cycle of mtHsp70.
Frazier, A. E. et al. Pam16 has an essential role in the mitochondrial protein import motor. Nature Struct. Mol. Biol. 11, 226–233 (2004).
Kozany, C., Mokranjac, D., Sichting, M., Neupert, W. & Hell, K. The J-domain related co-chaperone Tim16 is a constituent of the mitochondrial TIM23 preprotein translocase. Nature Struct. Mol. Biol. 11, 234–241 (2004). References 97 and 98 show that Pam16 (Tim16) is part of the PAM complex and is necessary for matrix-protein import. Pam16 is crucial for the association of Pam18 with the presequence translocase.
Zimmermann, R. & Neupert, W. Transport of proteins into mitochondria. Posttranslational transfer of ADP/ATP carrier into mitochondria in vitro. Eur. J. Biochem. 109, 217–229 (1980).
Zwizinski, C., Schleyer, M. & Neupert, W. Transfer of proteins into mitochondria. Precursor to the ADP/ATP carrier binds to receptor sites on isolated mitochondria. J. Biol. Chem. 258, 4071–4074 (1983).
Rassow, J. & Pfanner, N. Mitochondrial preproteins en route from the outer membrane to the inner membrane are exposed to the intermembrane space. FEBS Lett. 293, 85–88 (1991).
Schägger, H. & von Jagow, G. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal. Biochem. 199, 223–231 (1991).
Dekker, P. J., Müller, H., Rassow, J. & Pfanner, N. Characterization of the preprotein translocase of the outer mitochondrial membrane by blue native electrophoresis. Biol. Chem. 377, 535–538 (1996).
Dekker, P. J. T. et al. The Tim core complex defines the number of mitochondrial translocation contact sites and can hold arrested preproteins in the absence of matrix Hsp70–Tim44. EMBO J. 16, 5408–5419 (1997).
Johnston, A. J. et al. Insertion and assembly of human Tom7 into the preprotein translocase complex of the outer mitochondrial membrane. J. Biol. Chem. 277, 42197–42204 (2002).
Yang, T. et al. Crucial step in cholesterol homeostasis: sterols promote binding of SCAP to INSIG-1, a membrane protein that facilitates retention of SREBPs in ER. Cell 110, 489–500 (2002).
Stuart, R. Insertion of proteins into the inner membrane of mitochondria: the role of the Oxa1 complex. Biochim. Biophys. Acta 1592, 79–87 (2002).
Acknowledgements
We are grateful to A. E. Frazier for critical comments on the manuscript. Work from the authors' laboratory is supported by the Deutsche Forschungsgemeinschaft, the Sonderforschungsbereich 388 Freiburg, Max Planck Research Award, Alexander von Humboldt Foundation, Bundesministerium für Bildung und Forschung and the Fonds der Chemischen Industrie.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- MEMBRANE POTENTIAL
-
The mitochondrial respiratory chain generates a proton gradient across the inner mitochondrial membrane that leads to the formation of an electrochemical potential. This potential is comprised of two components — a chemical potential (ΔpH) and an electrical membrane potential (Δψ). Δψ is the part of the electrochemical potential that functions as a driving force in protein translocation.
- HSP90 CHAPERONES
-
Homodimer-forming chaperones that are composed of subunits that have a molecular mass of ∼90 kDa. Each subunit has an ATPase domain. Hsp90 proteins recognize specific substrate proteins and stabilize intermediate folding states of the protein.
- HSP70 CHAPERONES
-
Molecular chaperones of about 70 kDa that are composed of a substrate-binding domain and an ATPase domain. Hsp70 molecules interact with hydrophobic segments in unfolded proteins in an ATP-dependent manner and assist in protein folding through consecutive rounds of substrate binding and release.
- ZINC-FINGER MOTIF
-
A motif in proteins that contains conserved cysteine residues. The sulphydryl groups of the cysteines coordinate a Zn2+ ion.
- DNAJ-LIKE PROTEINS
-
Proteins that show similarity to a portion of the Escherichia coli DnaJ protein (the so-called J-domain) and that activate the ATPase activity of Hsp70 chaperones. The tripeptide His-Pro-Asp (HPD motif) is crucial for the ATPase-stimulating activity of the J-domain.
- IONOPHORES
-
Molecules that bind ions and that allow their passage across a membrane barrier by surrounding the charges during the passage of the ion across the lipophilic phase. Ionophores are usually specific for a defined set of ions — for example, protonophores are ionophores that are specific for H+.
- ELECTROPHORETIC FORCE
-
Movement of a protein across the inner mitochondrial membrane can be driven by the electric field that is generated by the membrane potential (Δψ). Positive charges in the protein move towards the negatively charged side of the membrane due to the driving force of the field.
Rights and permissions
About this article
Cite this article
Rehling, P., Brandner, K. & Pfanner, N. Mitochondrial import and the twin-pore translocase. Nat Rev Mol Cell Biol 5, 519–530 (2004). https://doi.org/10.1038/nrm1426
Issue Date:
DOI: https://doi.org/10.1038/nrm1426